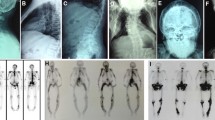
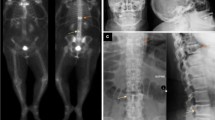
Abstract
The objective of this study is to report a Brazilian patient and his family with inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD). A systematic review of the literature on the valosin-containing protein (VCP) mutation was also performed. The proband (patient) was initially treated as a case of possible refractory polymyositis with Paget’s disease and later as an inclusion body myopathy. However, after admission to our service, and considering his personal and familial antecedents, whole exome sequencing was performed revealing valosin-containing protein (VCP) c.290G>A (p.Gly97Glu) mutation in the patient and his nine family members. The clinical presentation of the patient and his family was characterized by different degrees and evaluations of IBMPFD. According to the literature, only one family (Chinese) has this same VCP mutation concomitantly with different IBMPFD phenotype manifestations. The present study shows that IBMPFD should be considered as a differential diagnosis in patients with inflammatory myopathies associated to bone disease and/or cognitive impairment. Moreover, the study expands the genotypic spectrum of missense mutations of VCP gene in a Brazilian family with variable phenotypes.




Similar content being viewed by others
References
Kovach MJ, Waggoner B, Leal SM, Gelber D, Khardori R, Levenstien MA, Shanks CA, Gregg G, Al-Lozi MT, Miller T, Rakowicz W, Lopate G, Florence J, Glosser G, Simmons Z, Morris JC, Whyte MP, Pestronk A, Kimonis VE (2001) Clinical delineation and localization to chromosome 9p13.3–p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 74(4):458–475. https://doi.org/10.1006/mgme.2001.3256
Watts GDJ, Wymer K, Kovach MJ et al (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36(4):377–381. https://doi.org/10.1038/ng1332
Meyer H, Weihl CC (2014) The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci 127(18):3877–3883. https://doi.org/10.1242/jcs.093831
Rohrer JD, Warren JD, Reiman D, Uphill J, Beck J, Collinge J, Rossor MN, Isaacs AM, Mead S (2011) A novel exon 2 I27V VCP variant is associated with dissimilar clinical syndromes. J Neurol 258(8):1494–1496. https://doi.org/10.1007/s00415-011-5966-4
Abrahao A, Abath Neto O, Kok F, Zanoteli E, Santos B, Pinto WBVR, Barsottini OGP, Oliveira ASB, Pedroso JL (2016) One family, one gene and three phenotypes: a novel VCP (valosin-containing protein) mutation associated with myopathy with rimmed vacuoles, amyotrophic lateral sclerosis and frontotemporal dementia. J Neurol Sci 368:352–358. https://doi.org/10.1016/j.jns.2016.07.048
Krause S, Gohringer T, Walter MC, Schoser BG (2007) Brain imaging and neuropsychology in late-onset dementia due to a novel mutation (R93C) of valosin-containing protein. Clin Neuropathol 26(09):232–240. https://doi.org/10.5414/NPP26232
Hubbers CU, Clemen CS, Kesper K, Boddrich A, Hofmann A, Kamarainen O, Tolksdorf K, Stumpf M, Reichelt J, Roth U, Krause S, Watts G, Kimonis V, Wattjes MP, Reimann J, Thal DR, Biermann K, Evert BO, Lochmuller H, Wanker EE, Schoser BGH, Noegel AA, Schroder R (2007) Pathological consequences of VCP mutations on human striated muscle. Brain 130(2):381–393. https://doi.org/10.1093/brain/awl238
Bersano A, Del Bo R, Lamperti C, Ghezzi S, Fagiolari G, Fortunato F, Ballabio E, Moggio M, Candelise L, Galimberti D, Virgilio R, Lanfranconi S, Torrente Y, Carpo M, Bresolin N, Comi GP, Corti S (2009) Inclusion body myopathy and frontotemporal dementia caused by a novel VCP mutation. Neurobiol Aging 30(5):752–758. https://doi.org/10.1016/j.neurobiolaging.2007.08.009
Viassolo V, Previtali SC, Schiatti E, Magnani G, Minetti C, Zara F, Grasso M, Dagna-Bricarelli F, di Maria E (2008) Inclusion body myopathy, Paget’s disease of the bone and frontotemporal dementia: recurrence of the VCP R155H mutation in an Italian family and implications for genetic counselling. Clin Genet 74(1):54–60. https://doi.org/10.1111/j.1399-0004.2008.00984.x
Guyant-Marechal L, Laquerriere A, Duyckaerts C, Dumanchin C, Bou J, Dugny F, le Ber I, Frebourg T, Hannequin D, Campion D (2006) Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology 67(4):644–651. https://doi.org/10.1212/01.wnl.0000225184.14578.d3
Fanganiello RD, Kimonis VE, Corte CC, Nitrini R, Passos-Bueno MR (2011) A Brazilian family with hereditary inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Braz J Med Biol Res 44(4):374–380. https://doi.org/10.1590/S0100-879X2011007500028
Figueroa-Bonaparte S, Hudson J, Barresi R, Polvikoski T, Williams T, Töpf A, Harris E, Hilton-Jones D, Petty R, Willis TA, Longman C, Dougan CF, Parton MJ, Hanna MG, Quinlivan R, Farrugia ME, Guglieri M, Bushby K, Straub V, Lochmüller H, Evangelista T (2016) Mutational spectrum and phenotypic variability of VCP-related neurological disease in the UK. J Neurol Neurosurg Psychiatry 87(6):680–681. https://doi.org/10.1136/jnnp-2015-310362
Kimonis VE, Fulchiero E, Vesa J, Watts G (2008) VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim Biophys Acta 1782(12):744–774. https://doi.org/10.1016/j.bbadis.2008.09.003
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442(7105):916–919. https://doi.org/10.1038/nature05016
Kaleem M, Zhao A, Hamshere M, Myers AJ (2007) Identification of a novel valosin-containing protein polymorphism in late-onset Alzheimer’s disease. Neurodegener Dis 4(5):376–381. https://doi.org/10.1159/000105158
Jerath NU, Crockett CD, Moore SA, Shy ME, Weihl CC, Chou T-F, Grider T, Gonzalez MA, Zuchner S, Swenson A (2015) Rare manifestation of a c.290 CNT, p.Gly97Glu VCP mutation. Case Rep Genet 2015:239167. https://doi.org/10.1155/2015/239167
Gu JM, Ke YH, Yue H et al (2013) A novel VCP mutation as the cause of atypical IBMPFD in a Chinese family. Bone 52:9–16
Gidaro T, Modoni A, Sabatelli M, Tasca G, Broccolini A, Mirabella M (2008) An Italian family with inclusion-body myopathy and frontotemporal dementia due to mutation in the VCP gene. Muscle Nerve 37(1):111–114. https://doi.org/10.1002/mus.20890
Palmio J, Sandell S, Suominen T, Penttilä S, Raheem O, Hackman P, Huovinen S, Haapasalo H, Udd B (2011) Distinct distal myopathy phenotype caused by VCP gene mutation in a Finnish family. Neuromuscul Disord 21(8):551–555. https://doi.org/10.1016/j.nmd.2011.05.008
Vesa J, Su H, Watts GD, Krause S, Walter MC, Martin B, Smith C, Wallace DC, Kimonis VE (2009) Valosin containing protein associated inclusion body myopathy: abnormal vacuolization, autophagy and cell fusion in myoblasts. Neuromuscul Disord 19(11):766–772. https://doi.org/10.1016/j.nmd.2009.08.003
Schroder R, Watts GD, Mehta SG et al (2005) Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol 57(3):457–461. https://doi.org/10.1002/ana.20407
Mehta SG, Khare M, Ramani R, Watts GDJ, Simon M, Osann KE, Donkervoort S, Dec E, Nalbandian A, Platt J, Pasquali M, Wang A, Mozaffar T, Smith CD, Kimonis VE (2013) Genotype phenotype studies of VCP-associated inclusion body myopathy with Paget disease of bone and/or frontotemporal dementia. Clin Genet 83(5):422–431. https://doi.org/10.1111/cge.12000
Kim E-J, Park Y-E, Kim D-S, Ahn BY, Kim HS, Chang YH, Kim SJ, Kim HJ, Lee HW, Seeley WW, Kim S (2011) Inclusion body myopathy with Paget disease of bone and frontotemporal dementia linked to VCP p.Arg155Cys in a Korean family. Arch Neurol 68(6):787–796. https://doi.org/10.1001/archneurol.2010.376
Jacquin A, Rouaud O, Soichot P, Bejot Y, Dygai-Cochet I, Sarazin M, Stojkovic T, Lemesle-Martin M, Giroud M, Moreau T (2013) Psychiatric presentation of frontotemporal dementia associated with inclusion body myopathy due to the VCP mutation (R155H) in a French family. Case Rep Neurol 5(3):187–194. https://doi.org/10.1159/000356481
Djamshidian A, Schaefer J, Haubenberger D, Stogmann E, Zimprich F, Auff E, Zimprich A (2009) A novel mutation in the VCP gene (G157R) in a German family with inclusion-body myopathy with Paget disease of bone and frontotemporal dementia. Muscle Nerve 39(3):389–391. https://doi.org/10.1002/mus.21225
Spina S, Van Laar AD, Murrel JR et al (2008) Frontotemporal dementia associated with a valosincontaining protein mutation: report of three families. The FASEB J 22:251–258
Haubenberger D, Bittner RE, Rauch-Shorny S, Zimprich F, Mannhalter C, Wagner L, Mineva I, Vass K, Auff E, Zimprich A (2005) Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology 65(8):1304–1305. https://doi.org/10.1212/01.wnl.0000180407.15369.92
van der Zee J, Pirici D, Van Langenhove T, Engelborghs S, Vandenberghe R, Hoffmann M, Pusswald G, van den Broeck, Peeters K, Mattheijssens M, Martin JJ, de Deyn PP, Cruts M, Haubenberger D, Kumar-Singh S, Zimprich A, van Broeckhoven C (2009) Clinical heterogeneity in 3 unrelated families linked to VCP. Arg159His. Neurology 73(8):626–632. https://doi.org/10.1212/WNL.0b013e3181b389d9
Segers K, Glibert G, Callebaut J, Kevers L, Alcan I, Dachy B (2014) Involvement of peripheral and central nervous systems in a valosin-containing protein mutation. J Clin Neurol 10(2):166–175. https://doi.org/10.3988/jcn.2014.10.2.166
Spina S, Van Laar AD, Murrell JR, Hamilton RL, Kofler JK, Epperson F, Farlow MR, Lopez OL, Quinlan J, DeKosky ST, Ghetti B (2013) Phenotypic variability in three families with valosin-containing protein mutation. Eur J Neurol 20(2):251–258. https://doi.org/10.1111/j.1468-1331.2012.03831.x
Watts GD, Thomasova D, Ramdeen SK et al (2007) Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clin Genet 72(5):420–426. https://doi.org/10.1111/j.1399-0004.2007.00887.x
Wang H, Wu S (2015) Novel valosin containing protein mutation in a Swiss family with hereditary inclusion body myopathy and dementia. Neuromuscul Disord 25(3):273. https://doi.org/10.1016/j.nmd.2014.10.007
(1943) Aids to investigation of peripheral nerve injuries. Medical Research Council War Memorandum, revised 2nd edn. HMSO, London
Freitas S, Simões MR, Alves L, Duro D, Santana I (2012) Montreal Cognitive Assessment (MoCA): validation study for frontotemporal dementia. J Geriatr Psychiatry Neurol 25(3):146–154. https://doi.org/10.1177/0891988712455235
Miller SA, Dykes DD, Polesky HF (1998) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinforma Oxf Engl 25(14):1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Tischler G, Leonard S (2014) Biobambam: tools for read pair collation based algorithms on BAM files. Source Code Biol Med 9(1):13. https://doi.org/10.1186/1751-0473-9-13
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303. https://doi.org/10.1101/gr.107524.110
Wang K, Li M, Hakonarson H (2010) Annovar: functional annotation of genetic variants from next-generation sequencing data. Nucleic Acids Research 38:e16416. https://doi.org/10.1093/nar/gkq603
Guinto JB, Ritson GP, Taylor JP, Forman MS (2007) Valosin-containing protein and the pathogenesis of frontotemporal dementia associated with inclusion body myopathy. Acta Neuropathol 114:51–61
Weihl CC, Pestronk A, Kimonis VE (2009) Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord 19(5):308–315. https://doi.org/10.1016/j.nmd.2009.01.009
Shi Z, Hayashi YK, Mitsuhashi S, Goto K, Kaneda D, Choi YC, Toyoda C, Hieda S, Kamiyama T, Sato H, Wada M, Noguchi S, Nonaka I, Nishino I (2012) Characterization of the Asian myopathy patients with VCP mutations. Eur J Neurol 19(3):501–509. https://doi.org/10.1111/j.1468-1331.2011.03575.x
Nalbandian A, Donkervoort S, Dec E, Badadani M, Katheria V, Rana P, Nguyen C, Mukherjee J, Caiozzo V, Martin B, Watts GD, Vesa J, Smith C, Kimonis VE (2011) The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget’s disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis. J Mol Neurosci 45(3):522–531. https://doi.org/10.1007/s12031-011-9627-y
Meyer H, Bug M, Bremer S (2012) Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 14(2):117–123. https://doi.org/10.1038/ncb2407
Dai RM, Li CC (2001) Valosin-containing protein is a multi-ubiquitin chain targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol 3(8):740–744. https://doi.org/10.1038/35087056
Hirabayashi M, Inoue K, Tanaka K, Nakadate K, Ohsawa Y, Kamei Y, Popiel AH, Sinohara A, Iwamatsu A, Kimura Y, Uchiyama Y, Hori S, Kakizuka A (2001) VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ 8(10):977–984. https://doi.org/10.1038/sj.cdd.4400907
Wang Q, Song C, Li CC (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol 146:44–57, 1-2. https://doi.org/10.1016/j.jsb.2003.11.014
Acknowledgments
S.K.S, S.M.O.S, and S.K.N.M would like to thank the Faculdade de Medicina da Univesidade de Sao Paulo; S.K.S would like to thank Frederico Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The proband’s family gave its informed consent prior to its inclusion in the present study.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Shinjo, S.K., Oba-Shinjo, S.M., Lerario, A.M. et al. A Brazilian family with inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia linked to the VCP pGly97Glu mutation. Clin Rheumatol 37, 1129–1136 (2018). https://doi.org/10.1007/s10067-017-3913-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3913-1