Abstract
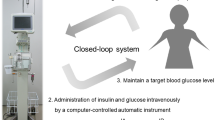
Although tight glucose control might reduce inflammation after cardiac surgery, it remains unclear whether inflammation can be controlled by maintaining glucose levels within 110–180 mg/dL. We hypothesized that a glucose target range of 110–180 mg/dL decreases inflammation after cardiovascular surgery. This retrospective study included 72 cardiovascular surgery patients divided into two groups according to the glucose control approach. Patients allocated to the closed-loop group received closed-loop glucose control (target glucose levels at 110–180 mg/dL) from admission to the intensive care unit until 9 a.m. on postoperative day (POD) 1. Patients allocated to the conventional group received conventional glucose control using a sliding scale method to maintain blood glucose levels < 200 mg/dL. Primary outcomes were C-reactive protein (CRP) levels on PODs 1, 2, and 7. Data were reported as mean ± standard deviation. Comparisons were performed using the chi-squared test and unpaired t test, with p < 0.05 indicating statistical significance. The closed-loop group had significantly lower average glucose levels (169 ± 24 vs. 201 ± 36 mg/dL, p < 0.001) and standard deviation of glucose levels (22 ± 13 vs. 44 ± 20 mg/dL; p < 0.001). The CRP levels on PODs 2 and 7 were significantly lower in the closed-loop group than in the conventional group (10.8 ± 5.6 vs. 14.1 ± 5.7 mg/dL, p = 0.02; 4.6 ± 2.5 vs. 7.3 ± 4.0 mg/dL, p < 0.001; respectively). Our findings suggest that glucose control using a closed-loop device might decrease inflammation after cardiovascular surgery without increasing hypoglycemia risk.

Similar content being viewed by others
References
Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Rizzo MR, Siniscalchi M, Musacchio E, D’Amico M, Rossi F, Paolisso G. Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients. J Am Coll Cardiol. 2009;534:1425–36.
Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74.
Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497–502.
Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, Damiano RJ Jr. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–8.
Yatabe T, Yamazaki R, Kitagawa H, Okabayashi T, Yamashita K, Hanazaki K, Yokoyama M. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575–8.
Hasegawa A, Iwasaka H, Hagiwara S, Koga H, Hasegawa R, Kudo K, Kusaka J, Noguchi T. Anti-inflammatory effects of perioperative intensive insulin therapy during cardiac surgery with cardiopulmonary bypass. Surg Today. 2011;41:1385–90.
Yatabe T, Inoue S, Sakaguchi M, Egi M. The optimal target for acute glycemic control in critically ill patients: a network meta-analysis. Intensive Care Med. 2017;43:16–28.
Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T, Imaizumi H, Inoue S, Kakihana Y, Kotani J, Kushimoto S, Masuda Y, Matsuda N, Matsushima A, Nakada TA, Nakagawa S, Nunomiya S, Sadahiro T, Shime N, Yatabe T, Hara Y, Hayashida K, Kondo Y, Sumi Y, Yasuda H, Aoyama K, Azuhata T, Doi K, Doi M, Fujimura N, Fuke R, Fukuda T, Goto K, Hasegawa R, Hashimoto S, Hatakeyama J, Hayakawa M, Hifumi T, Higashibeppu N, Hirai K, Hirose T, Ide K, Kaizuka Y, Kan’o T, Kawasaki T, Kuroda H, Matsuda A, Matsumoto S, Nagae M, Onodera M, Ohnuma T, Oshima K, Saito N, Sakamoto S, Sakuraya M, Sasano M, Sato N, Sawamura A, Shimizu K, Shirai K, Takei T, Takeuchi M, Takimoto K, Taniguchi T, Tatsumi H, Tsuruta R, Yama N, Yamakawa K, Yamashita C, Yamashita K, Yoshida T, Tanaka H, Oda S. The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J-SSCG 2016). J Intensive Care. 2018;6:7.
Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403.
Vlasselaers D, Mesotten D, Langouche L, Vanhorebeek I, van den Heuvel I, Milants I, Wouters P, Wouters P, Meyns B, Bjerre M, Hansen TK, Van den Berghe G. Tight glycemic control protects the myocardium and reduces inflammation in neonatal heart surgery. Ann Thorac Surg. 2010;90:22–9.
Lebherz C, Kahles F, Piotrowski K, Vogeser M, Foldenauer AC, Nassau K, Kilger E, Marx N, Parhofer KG, Lehrke M. Interleukin-6 predicts inflammation-induced increase of glucagon-like peptide-1 in humans in response to cardiac surgery with association to parameters of glucose metabolism. Cardiovasc Diabetol. 2016;15:21.
Reyes-Umpierrez D, Davis G, Cardona S, Pasquel FJ, Peng L, Jacobs S, Vellanki P, Fayfman M, Haw S, Halkos M, Guyton RA, Thourani VH, Umpierrez GE. Inflammation and oxidative stress in cardiac surgery patients treated to intensive versus conservative glucose targets. J Clin Endocrinol Metab. 2017;102:309–15.
Sato H, Hosojima M, Ishikawa T, Aoki K, Okamoto T, Saito A, Tsuchida M. Glucose variability based on continuous glucose monitoring assessment is associated with postoperative complications after cardiovascular surgery. Ann Thorac Cardiovasc Surg. 2017;23:239–47.
Yoo S, Lee HJ, Lee H, Ryu HG. Association between perioperative hyperglycemia or glucose variability and postoperative acute kidney injury after liver transplantation: a retrospective observational study. Anesth Analg. 2017;124:35–41.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tamura, T., Yatabe, T., Namikawa, T. et al. Glucose control using a closed-loop device decreases inflammation after cardiovascular surgery without increasing hypoglycemia risk. J Artif Organs 22, 154–159 (2019). https://doi.org/10.1007/s10047-018-1082-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1082-x