Abstract
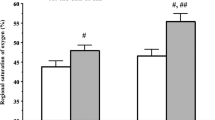
Near-infrared spectroscopy has been used to measure regional saturation of oxygen (rSO2) based on the total hemoglobin (t-Hb) signal strength. To date, few studies have investigated the changes of systemic oxygenation and t-Hb signal strength during hemodialysis (HD). This study aimed to (1) monitor rSO2 and t-Hb signal strength in the brain, liver, and lower-limb muscle during HD and (2) clarify the differences in rSO2 and t-Hb signal strength in each compartment. Fifty-three patients receiving 4-h HD were included and divided into three groups according to the compartments in which tissue oxygenation was measured as follows: brain (n = 44), liver (n = 42), and lower-limb muscle (n = 40). The rSO2 and t-Hb signal strength was monitored using an INVOS 5100c (Covidien Japan, Tokyo, Japan). The rSO2 levels were significantly lower in the brain than in the liver from HD initiation to the end (HD initiation: rSO2 in the brain and liver, 46.5 ± 1.3 and 52.4 ± 1.7%, respectively, p = 0.031). Furthermore, compared to the t-Hb signal strength ratio [value at t (min) during HD/initial value before HD] in the brain during HD, there were significant increases in the liver and lower-limb muscle, respectively. In conclusion, deterioration of cerebral oxygenation was remarkable compared to the hepatic oxygenation in HD patients. Our results, which revealed significant differences among the t-Hb signal strength ratios in the brain, liver, and lower-limb muscle during HD, might reflect the non-uniform body-fluid reduction within systemic tissues induced by ultrafiltration.




Similar content being viewed by others
References
Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–43.
Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–87.
Parnia S, Nasir A, Ahn A, Malik H, Yang J, Zhu J, Dorazi F, Richman P. A feasibility study of cerebral oximetry during in-hospital mechanical and manual cardiopulmonary resuscitation. Crit Care Med. 2014;42:930–3.
Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, Brown C, Katz NM, Grams ME, Hogue CW. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–71.
McCusker K, Chalafant A, de Foe G, Gunaydin S, Vijay V. Influence of hematocrit and pump prime on cerebral oxygen saturation in on-pump coronary revascularization. Perfusion. 2006;21:149–55.
Calderon-Arnulphi M, Alaraj A, Amin-Hanjani S, Mantulin WW, Polzonetti CM, Gratton E, Charbel FT. Detection of cerebral ischemia in neurovascular surgery using quantitative frequency-domain near-infrared spectroscopy. J Neurosurg. 2007;106:283–90.
Boezeman RP, Kelder JC, Waanders FG, Moll FL, de Vries JP. In vivo measurements of regional hemoglobin oxygen saturation values and limb-to-arm ratios of near-infrared spectroscopy for tissue oxygenation monitoring of lower extremities in healthy subjects. Med Devices (Auckl). 2014;8:31–6.
Shuller MS, Reisman WM, Kinsey TL, Whitesides TE Jr, Hammerberg EM, Davila MG, Moore TJ. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92:863–70.
Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:83–7.
Schat TE, Schurink M, van der Laan ME, Hulscher JBF, Hulzebos CV, Bos AF, Kooi EM. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS One. 2016;11:e0154710. doi:10.1371/journal.pone.0154710.
Ito K, Ookawara S, Ueda Y, Goto S, Miyazawa H, Yamada H, Kitano T, Shindo M, Kaku Y, Hirai K, Yoshida M, Hoshino T, Nabata A, Mori H, Yoshida I, Kakei M, Tabei K. Factors affecting cerebral oxygenation in hemodialysis patients: cerebral oxygenation associates with pH, hemodialysis duration, serum albumin concentration, and diabetes mellitus. PLoS One. 2015;10:e0117474. doi:10.1371/journal.pone.0117474.
Miyazawa H, Ookawara S, Ito K, Yanai K, Ishii H, Kitano T, Shindo M, Ueda Y, Kaku Y, Hirai K, Hoshino T, Tabei K, Morishita Y. Factors associating with oxygenation of lower-limb muscle tissue in hemodialysis patients. World J Nephrol. 2016;5:524–30. doi:10.5527/wjn.v5.i6.524.
Myers D, McGraw M, George M, Mulier K, Beilman G. Tissue hemoglobin index: a non-invasive optical measure of total tissue hemoglobin. Crit Care. 2009;13(suppl 5):S2. doi:10.1186/cc8000.
Ueda S, Nakamiya N, Matsuura K, Shigekawa T, Sano H, Hirokawa E, Shimada H, Suzuki H, Oda M, Yamashita Y, Kishino O, Kuji I, Osaki A, Saeki T. Optical imaging of tumor vascularity associated with proliferation and glucose metabolism in early breast cancer: clinical application of total hemoglobin measurements in the breast. BMC Cancer. 2013;13:514. doi:10.1186/1471-2407-13-514.
Nielsen AL, Thunedborg P, Brinkenfeldt H, Hegbrant J, Jensen HA, Wandrup JH. Assessment of pH and oxygen status during hemodialysis using the arterial blood line in patients with an arteriovenous fistula. Blood Purif. 1999;17:206–12.
Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–13.
Yoshida I, Ando K, Ando Y, Ookawara S, Suzuki M, Furuya H, Iimura O, Takeda D, Kajiya M, Komada T, Mori H, Tabei K, BVM study Group. A new device to monitor blood volume in hemodialysis patients. Ther Apher Dial. 2010;14:560–5.
Lemmers PMA, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–7.
Hyttel-Sorensen S, Sorensen LC, Riera J, Greisen G. Tissue oximetry: a comparison of mean values of regional tissue saturation, reproducibility and dynamic range of four NIRS-instruments on the human forearm. Biomed Opt Express. 2011;2:3047–57.
Schmitz J, Pichler G, Schwaberger B, Urlesberger B, Baik N, Binder C. Feasibility of long-term cerebral and peripheral regional tissue oxygen saturation measurements. Physiol Meas. 2014;35:1349–55.
Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F. Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neuro Res. 1995;17:89–93.
Maslehaty H, Krause-Tilz U, Petridis AK, Barth H, Mehdorn HM. Continuous measurement of cerebral oxygenation with near-infrared spectroscopy after spontaneous subarachnoid hemorrhage. ISRN Neurol 2012;907187. doi:10.5402/2012/907187.
Ookawara S, Suzuki M, Yahagi T, Saitou M, Tabei K. Effect of postural change on blood volume in long-term hemodialysis patients. Nephron. 2001;87:27–34.
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Apply Physiol. 1994;77:2740–7.
Song JG, Jeon SM, Shin WJ, Jun IG, Shin K, Huh IY, Kim YK, Hwang GS. Laboratory variables associated with low near-infrared cerebral oxygen saturation in icteric patients before liver transplantation surgery. Anesth Analg. 2011;112:1347–52.
Goto M, Kawano S, Yoshihara H, Takei Y, Hijioka T, Fukui H, Matsunaga T, Oshita M, Kashiwagi T, Fusamoto H. Hepatic tissue oxygenation as a predictive indicator of ischemia-reperfusion liver injury. Hepatology. 1992;15:432–7.
El-Desoky AE, Jiao LR, Havlik R, Habib N, Davidson BR, Seifalian AM. Measurement of hepatic tissue hypoxia using near infrared spectroscopy: comparison with hepatic vein oxygen partial pressure. Eur Surn Res. 2000;32:207–14.
Saad WEA. Nonocclusive hepatic artery hypoperfusion syndrome (splenic steal syndrome) in liver transplant recipients. Semin Intervent Radiol. 2012;29:140–6.
Bailey SM, Hendrics-Munoz KD, Mally P. Splanchnic-cerebral oxygenation ratio as a marker of preterm infant blood transfusion needs. Transfusion. 2012;52:252–60.
Bailey SM, Hendrics-Munoz KD, Mally P. Splanchnic-cerebral oxygenation ratio (SCOR) values in healthy term infants as measured by near-infrared spectroscopy. Pediatr Surg Int. 2013;29:591–5.
Dantsker DR. The gastrointestinal tract: the canary of the body? JAMA. 1993;270:1247–8.
Taylor J, Mulier K, Myers D, Beilman GJ. Use of infrared spectroscopy in early determination of irreversible hemorrhagic shock. J Trauma. 2005;58:119–1125.
Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis. 2001;38:S11–7.
Dasselaar JJ, van der Sande FM, Franssen CFM. Critical evaluation of blood volume measurements during hemodialysis. Blood Purif. 2012;33:177–82.
Ishida I, Hirakata H, Sugimori H, Omae T, Hirakata E, Ibayashi S, Kubo M, Fujisawa M. Hemodialysis causes severe orthostatic reduction in cerebral blood flow velocity in diabetic patients. Am J Kidney Dis. 1999;34:1096–104.
Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, Tanaka H, Kanai H, Fujimi S, Iida M. Dialysis-related hypotension as a cause for progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97:c23–30.
Miyazawa H, Ookawara S, Tabei K. Aggravation of cerebral oxygenation due to intradialytic hypotension induced by blood volume reduction during hemodialysis: a case report. Ther Apher Dial. 2015;19:525–7.
Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Wen W, Zagami AS. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–9.
Acknowledgements
We thank the study participants and our hospital’s clinical dialysis center staff.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ookawara, S., Ito, K., Ueda, Y. et al. Differences in tissue oxygenation and changes in total hemoglobin signal strength in the brain, liver, and lower-limb muscle during hemodialysis. J Artif Organs 21, 86–93 (2018). https://doi.org/10.1007/s10047-017-0978-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-017-0978-1