Abstract
Introduction
We evaluated the usefulness of xeno-Biosheets, an in-body tissue architecture-induced bovine collagenous sheet, as repair materials for abdominal wall defects in a beagle model.
Materials and Methods
Biosheets were prepared by embedding cylindrical molds into subcutaneous pouches of three Holstein cows for 2–3 months and stored in 70% ethanol. The Biosheets were 0.5 mm thick, cut into 2 cm × 2 cm, and implanted to replace defects of the same size in the abdominal wall of nine beagles. The abdominal wall and Biosheets were harvested and subjected to histological evaluation at 1, 3, and 5 months after implantation (n = 3 each).
Results
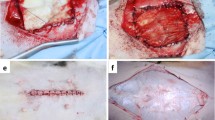
The Biosheet and bovine pericardiac patch (control) were not stressed during the suture operation and did not split, and patches were easily implanted on defective wounds. After implantation, the patch did not fall off and was not perforated, and healing was observed nacroscopically in all cases. During the first month of implantation, accumulation of inflammatory cells was observed along with decomposition around the Biosheet. Decomposition was almost complete after 3 months, and the Biosheet was replaced by autologous collagenous connective tissue without rejection. After 5 months, the abdominal wall muscle elongated from the periphery of the newly formed collagen layer and the peritoneum was formed on the peritoneal cavity surface. Regeneration of almost all layers of the abdominal wall was observed. However, almost all pericardium patches were remained even at 5 months with inflammation.
Conclusion
Bovine Biosheets requiring no special post-treatment can be useful as off-the-shelf materials for abdominal wall repair.


Similar content being viewed by others
References
Wang ZL, Wu SZ, Li ZF, Guo JH, Zhang Y, Pi JK, Hu JG, Yang XJ, Huang FG, Xie HQ (2018) Comparison of small intestinal submucosa and polypropylene mesh for abdominal wall defect repair. J Biomater Sci Polym Ed 29:663–682
Akcakaya A, Aydogdu I, Citgez B (2018) Investigation into the optimal prosthetic material for wound healing of abdominal wall defects. Exp Ther Med 15:1622–1625
Lukasiewicz A, Drewa T (2014) Synthetic implants in hernia surgery. Adv Clin Exp Med 23:135–142
Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE (2017) Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg 224:341–350
Takahashi M, Ono K, Wakakuwa R, Sato O, Tsuchiya Y, Kamiya G, Nitta K, Tajima K, Wada K (1994) The use of a human dura mater allograft for the repair of a contaminated abdominal wall defect: report of a case. Surg Today 24:468–472
Lai PH, Chang Y, Liang HC, Chen SC, Wei HJ, Sung HW (2005) Peritoneal regeneration induced by an acellular bovine pericardial patch in the repair of abdominal wall defects. J Surg Res 127:85–92
Gurrado A, Franco IF, Lissidini G, Greco G, De Fazio M, Pasculli A, Girardi A, Piccinni G, Memeo V, Testini M (2015) Impact of pericardium bovine patch (Tutomesh(®)) on incisional hernia treatment in contaminated or potentially contaminated fields: retrospective comparative study. Hernia 19:259–266
Nakayama Y, Furukoshi M (2018) Feasibility of in-body tissue architecture in pediatric cardiovascular surgery: development of regenerative autologous tissues with growth potential. J Pediatr Cardiol Cardiac Surg 2:28–36
Ishii D, Enmi JI, Iwai R, Kurisu K, Tatsumi E, Nakayama Y (2018) One year rat study of iBTA-induced “microbiotube” microvascular grafts with an ultra-small diameter of 0.6 mm. Eur J Vasc Endovasc Surg 55:882–887
Takiyama N, Mizuno T, Iwai R, Uechi M, Nakayama Y (2016) In-body tissue-engineered collagenous connective tissue membranes (BIOSHEETs) for potential corneal stromal substitution. J Tissue Eng Regen Med 10:E518–E526
Suzuki K, Komura M, Terawaki K, Kodaka T, Gohara T, Komura H, Nakayama Y (2018) Engineering and repair of diaphragm using biosheet (a collagenous connective tissue membrane) in rabbits. J Pediatr Surg 53:330–334
Okuyama H, Umeda S, Takama Y, Terasawa T, Nakayama Y (2018) Patch esophagoplasty using an in-body-tissue-engineered collagenous connective tissue membrane. J Pediatr Surg 53:223–226
Takewa Y, Sumikura H, Kishimoto S, Naito N, Iizuka K, Akiyama D, Iwai R, Tatsumi E, Nakayama Y (2018) Implanted in-body tissue-engineered heart valve can adapt the histological structure to the environment. ASAIO J 64:395–405
Terazawa T, Nishimura T, Mitani T, Ichii O, Ikeda T, Kosenda K, Tatsumi E, Nakayama Y (2018) Wall thickness control in biotubes prepared using type-C mold. J Artif Organs. https://doi.org/10.1007/s10047-018-1035-4
Terazawa T, Kawashima T, Umeno T, Wada T, Tatsumi E, Ozaki S, Miyamoto S, Nakayama Y (2018) Material and mechanical properties of iBTA-induced autologous Biosheet for application as aortic valve reconstruction material. J Artif Organs (submitted)
Ozaki S, Kawase I, Yamashita H, Uchida S, Takatoh M, Kiyohara N (2018) Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg 155:2379–2387
Okamoto K, Kawashima T, Nakayama Y, Anai H, Wada T, Takebayashi S, Shuto T, Kozaki S, Miyamoto S (2016) Aortic valve reconstruction using in-body tissue-engineered collagenous connective tissue membranes, BIOSHEETs, in a 3-month goat model. Circulation 134:A13472
Lopesa MS, Jardinib AL, Filho RM (2012) Poly (lactic acid) production for tissue engineering applications. Procedia Eng 42:1402–1413
Uretzky G, Appelbaum Y, Younes H, Udassin R, Nataf P, Baccioglu E, Pizof G, Borman JB, Cohn D (1990) Long-term evaluation of a new selectively biodegradable vascular graft coated with polyethylene oxide-polylactic acid for right ventricular conduit. An experimental study. J Thorac Cardiovasc Surg 100:769–776
Yankah CA, Pasic M, Musci M, Stein J, Detschades C, Siniawski H, Hetzer R (2008) Aortic valve replacement with the Mitroflow pericardial bioprosthesis: durability results up to 21 years. J Thorac Cardiovasc Surg 136:688–696
Fisher O, Meecham L, Buxton P, Legge J, Fairhead J, Rajagopalan S, Asquith J, Pherwani A (2018) Long-term outcomes of bovine pericardial patch angioplasty for recurrent stenosis in vascular access: a UK single-centre experience. J Vasc Access. https://doi.org/10.1177/1129729818769795
Jorge-Herrero E, Garcia Paez JM, Del Castillo-Olivares Ramos JL (2005) Tissue heart valve mineralization: review of calcification mechanisms and strategies for prevention. J Appl Biomater Biomech 3:67–82
Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W (2011) Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg 92:1308–1314
Acknowledgements
We would like to thank Manami Sone, Tomohiro Mitani, Teppei Ikeda, and Keigo Kosenda for their valuable help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest associated with this work.
Ethical approval
The animal experiments were approved by the National Cerebral and Cardiovascular Center Research Institute Committee (No. 17013) and Hokkaido University (No. 16-0110).
Human and animal rights
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (NIH Publication No.85-23, received 1996).
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Nakayama, Y., Oshima, N., Tatsumi, E. et al. iBTA-induced bovine Biosheet for repair of abdominal wall defects in a beagle model: proof of concept. Hernia 22, 1033–1039 (2018). https://doi.org/10.1007/s10029-018-1799-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-018-1799-8