Abstract
Introduction
Ventral hernias are a common problem in a general surgery and hernioplasty is an integral part of a general surgeon’s practice. The use of prosthetic material has drastically reduced the risk of recurrence, but has introduced additional potential complications such as surgical wound infections, adhesion formation, graft rejection, etc. The development of a wound infection in a hernia that is repaired with a prosthetic material is a grave complication, often requiring removal of the prosthesis. This experimental study examined efficacy of completely absorbable, hydrophilic, PGA–TMC (polyglycolic acid–trimethylene carbonate) prosthesis impregnated with antibiotic for reduction of infectious complications.
Methods
Antibiotic-impregnated PGA–TMC prostheses were placed intraperitoneally in 90 Wistar white rats that were randomized and distributed into four groups. Group 0 (23 rats): there were placed PGA–TMC prosthesis without antibiotic impregnation (control group). Group 1 (25 rats): meshes were placed and infected later with 1 × 108 UFC of S. aureus/1 ml/2 cm2 (Staphylococcus aureus ATCC 6538 American Type Culture Collection, Rockville, MD). Group 2 (21 rats): cefazolin-impregnated prostheses were placed (1 g × 100 ml, at the rate of 1 ml/cm2 of prosthesis) and were subsequently infected with the same bacterial inoculate. Group 3 (21 rats): cefazolin-impregnated prostheses with double quantity of cefazolin and infected. A week later these animals were killed and specimens were extracted for bacterial quantification and histological studies.
Results
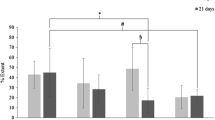
Evident decrease of bacterial colonization was observed in series 2 and 3 [the ones impregnated with cefazolin, in comparison with the group 1 (infected without previous antibiotic impregnation)] with statistically significant results (p < 0.00). Results were really positive when the antibiotic solution had been applied to the mesh. There have been formed adherences to the prosthesis when placing it in contact with intraabdominal viscera. However, cefazolin impregnation of the mesh has reduced an adhesion formation, mostly when the infection reached a minimum, inhibiting the inflammatory answer to the infection in a prosthetic material.
Conclusion
Impregnation of the absorbable hydrophilic prosthesis PGA–TMC with cefazolin prevents the infection of the prosthesis placed in infected localization. Therefore, we think this option should be considered as a new and useful alternative in case of contaminated and dirty surgical fields or when a replacement of the prosthesis is required.



Similar content being viewed by others
References
Zeller JL, Burke AE, Glass RM (2007) JAMA patient page. MRSA infections. JAMA 298(15):1826
Torrobal L, Rivero M, Otermin I, Gil A, Maraví-Poma E, García Irure JJ (2000) Antimicrobian resistance and antibiotics policy: MRSA, GISA and VRE. Anales Sis San Navarra 23(Suppl. 2):69–80
Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, Cornaby AJ (2001) Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 49(2):109–116
Conference Consensus (1984) Clinical applications of biomaterials. JAMA 1984(249):1050
Carbonell AM, Kercher KW, Sing RF, Heniford BT (2005) Susceptibility of prosthetic biomaterials to infection. Surg Endosc 19(12):1670
Amid PK (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15
Martin-Cartes J, Morales-Conde S, Suarez-Grau J, Lopez-Bernal F, Bustos-Jimenez M, Cadet-Dussort H, Socas-Macias M, Alamo-Martinez J, Tutosaus-Gomez JD, Morales-Mendez S (2008) Use of hyaluronidase cream to prevent peritoneal adhesions in laparoscopic ventral hernia repair by means of intraperitoneal mesh fixation using spiral tacks. Surg Endosc 22(3):631–634
Culver DH, Horan TC, Gaynes RP, National Nosocomial Infections Surveillance Systems (NNIS) (1991) Surgical wound infections rates by wound class, operation, and risk index in U.S. hospitals. Am J Med 91(Suppl. 3B):152–157
Schierholz JM, Beuth J (2001) Implant infections: a haven for opportunistic bacteria. J Hosp Infect 49(2):87–93
Taylor EW, Duffy K, Lee K, Hill R, Noone A, Macintyre I, King PM, O’Dwyer PJ (2004) Surgical site infection after groin hernia repair. Br J Surg 91(1):105–111
Falagas ME, Kasiakou SK (2005) Mesh-related infections after hernia repair surgery. Clin Microbiol Infect 11(1):3–8
Ott R, Hartwig T, Tannapfel A, Blatz R, Rodloff AC, Madaj-Sterba P, Möbius Ch, Köckerling F (2007) Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg 392(4):473–478
Suarez Grau JM, De Toro Crespo M, Docobo Durantez F, Rubio Chaves C, Martin Cartes JA, Docobo Perez F (2007) Prevention of surgical infection using reabsorbable antibacterial suture (Vicryl Plus) versus reabsorbable conventional suture in hernioplasty. An experimental study in animals. Cir Esp 81(6):324–329
Bellon JM, Garcia-Carranza A, Garcia-Honduvilla N, Carrera-San Martin A, Bujan J (2004) Tissue integration and biomechanical behaviour of contaminated experimental polypropylene and expanded polytetrafluoroethylene implants. Br J Surg 91(4):489–494
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63(6):765–771
Junge K, Rosch R, Klinge U, Krones C, Klosterhalfen B, Mertens PR, Lynen P, Kunz D, Preiss A, Peltroche-Llacsahuanga H, Schumpelick V (2005) Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 26(7):787–793
Agalar C, Ozdogan M, Agalar F, Saygun O, Aydinuraz K, Akkus A, Ceken S, Akturk S (2006) A rat model of polypropylene graft infection caused by Staphylococcus epidermidis. ANZ J Surg. 76(5):387–391
van’t Riet M, de Vos van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J (2007) Mesh repair for postoperative wound dehiscence in the presence of infection: is absorbable mesh safer than non-absorbable mesh? Hernia 11(5):409–413
Conflict of interest
JMSG declares no conflict of interest. SMC declares no conflict of interest. VGG declares no conflict of interest. JMC declares no conflict of interest. FDD declares no conflict of interest. FJPD declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suárez-Grau, J.M., Morales-Conde, S., González Galán, V. et al. Antibiotic embedded absorbable prosthesis for prevention of surgical mesh infection: experimental study in rats. Hernia 19, 187–194 (2015). https://doi.org/10.1007/s10029-014-1334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-014-1334-5