Abstract
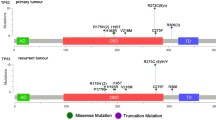
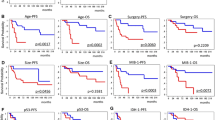
The genetic features of isocitrate dehydrogenase-wild-type (IDH-wt) lower-grade gliomas (LGGs; World Health Organization grades II and III) are not well defined. This study analyzed the genetic and other features of IDH-wt LGGs to develop a subclassification that can be used to predict their prognosis. Clinical, histopathological, and genetic features of 35 cases of diffuse IDH-wt astrocytoma and IDH-wt anaplastic astrocytoma were analyzed. The following genetic factors were examined: mutations of B-rapidly accelerated fibrosarcoma, telomerase reverse transcriptase promoter (TERTp), histone 3 family 3A, and alpha-thalassemia/mental retardation syndrome, X-linked; and copy number aberrations. In the univariate analysis, the following factors were associated with poor overall survival (OS): the histopathological diagnosis, TERTp mutation, the gain of chromosome 7 (+ 7), and the loss of chromosome 10q (− 10q). In the multivariate analysis, + 7, − 10q, and TERTp mutation were independent prognostic factors associated with poor OS. The median OS was significantly worse for patients who harbored at least one of these factors than for those without any of them (18.5 vs. 54.5 months, P = 0.002). The subclassification of IDH-wt LGGs according to the genetic factors + 7, − 10q, and TERTp mutation is potentially useful for predicting the prognosis.



Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD et al (2016) WHO classification of tumours of the central nervous system, 4th edn. IARC, Lyon
Wijnenga MMJ, Dubbink HJ, French PJ et al (2017) Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol 134:957–959
Aibaidula A, Chan AKY, Shi Z et al (2017) Adult IDH wild-type lower grade gliomas should be further stratified. Neuro Oncol 19(10):1327–1337
Weller M, Weber RG, Willscher E et al (2015) Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129:679–693
Stichel D, Ebrahimi A, Reuss D et al (2018) Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol 136(5):793–803
Brat DJ, Aldape K, Colman H et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol 136(5):805–810
Aoki K, Nakamura H, Suzuki H et al (2018) Prognostic relevance of genetic alterations in diffuse lower-grade glioma. Neuro Oncol 20(1):66–77
van den Bent MJ, Weller M, Wen PY et al (2017) A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro Oncol 19(5):614–624
Hirose Y, Sasaki H, Miwa T et al (2011) Whole genome analysis from microdissected tissue revealed adult supratentorial grade II–III gliomas are divided into clinically relevant subgroups by genetic plofiles. Neurosurgery 69(2):376–390
Hattori N, Hirose Y, Sasaki H et al (2016) World Health Organization grade II–III astrocytomas consist of genetically distinct tumor lineages. Cancer Sci 107(8):1159–1164
Arslantas A, Artan S, Oner U et al (2007) Genomic alterations in low-grade, anaplastic astrocytomas and glioblastomas. Pathol Oncol Res 13(1):39–46
Koelsche C, Sahm F, Capper D et al (2013) Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol 126(6):907–915
Liu XY, Gerges N, Korshunov A et al (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124(5):615–625
Schindler G, Capper D, Meyer J et al (2011) Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121:397–405
Dahiya S, Emnett RJ, Haydon DH et al (2014) BRAF-V600E mutation in pediatric and adult glioblastoma. Neuro Oncol 16(2):318–319
Pekmezci M, Rice T, Molinaro AM et al (2017) Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol 133(6):1001–1016
Bloch O, Han SJ, Cha S et al (2012) Impact of extent of resection for recurrent glioblastoma on overall survival. J Neurosurg 117:1032–1038
Wisoff JH, Boyett JM, Berger MS et al (1998) Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial no. CCG-945. J Neurosurg 89(1):52–59
Almenawer SA, Badhiwala JH, Alhazzani W et al (2015) Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol 17(6):868–881
Nakagawa Y, Sasaki H, Ohara K et al (2017) Clinical and molecular prognostic factors for long-term survival of patients with glioblastomas in single-institutional consecutive cohort. World Neurosurg 106:165–173
Liu X, Bishop J, Shan Y et al (2013) Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 20(4):603–610
Huang T, Zhuge J, Zhang W (2013) Sensitive detection of BRAF V600E mutation by amplification refractory mutation system (ARMS)-PCR. Biomark Res 1:3
Zhang R, Shi Z, Chen H et al (2015) Biomarker-based prognostic stratification of young adult glioblastoma. Oncotarget 7(4):5030–5041
Gielen GH, Gessi M, Hammes J et al (2013) H3F3A K27M mutation in pediatric CNS tumors a marker for diffuse high-grade astrocytomas. Am J Clin Pathol 139(3):345–349
Olar A, Wani KM, Alfaro-Munoz KD et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol 129(4):585–596
Hewer E, Vajtai I, Dettmer MS et al (2016) Combined ATRX/IDH immunohistochemistry predicts genotype of oligoastrocytomas. Histopathol 68:272–278
Wiestler B, Capper D, Holland-Letz T et al (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48(3):452–458
Pallud J, Fontaine D, Duffau H et al (2010) Natural history of incidental World Health Organization grade II gliomas. Ann Neurol 68(5):727–733
Reinhardt A, Stichel D, Schrimpf D et al (2018) Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol 136(2):273–291
Acknowledgements
We also thank Mrs. Fujiko Sueishi and Mrs. Tomoko Suzuki for technical support and Mr. Takeo Ezaki for technical advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuwahara, K., Ohba, S., Nakae, S. et al. Clinical, histopathological, and molecular analyses of IDH-wild-type WHO grade II–III gliomas to establish genetic predictors of poor prognosis. Brain Tumor Pathol 36, 135–143 (2019). https://doi.org/10.1007/s10014-019-00348-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-019-00348-9