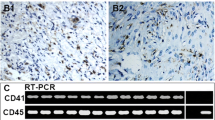
Abstract
Hemangioblastoma is composed of neoplastic stromal cells and a prominent capillary network. To date, the identity of stromal cells remains unclear. Mesenchymal stem cells can give rise to committed vascular progenitor cells, and ephrin-B2/EphB4 and Notch signaling have crucial roles in these steps. The aim of our study was to elucidate that stromal cells of central nervous system hemangioblastomas have mesenchymal stem cell-derived vascular progenitor cell properties. Ten hemangioblastomas were investigated immunohistochemically. CD44, a mesenchymal stem cell marker, was detected in stromal cells of all cases, suggesting that stromal cells have mesenchymal stem cell-like properties. Neither CD31 nor α-SMA was expressed in stromal cells, suggesting that stromal cells have not acquired differentiated vascular cell properties. Both ephrin-B2 and EphB4, immature vascular cell markers, were detected in stromal cells of all cases. Jagged1, Notch1, and Hesr2/Hey2, which are known to be detected in both immature endothelial cells and mural cells, were expressed in stromal cells of all cases. Notch3, which is known to be detected in differentiating mural cells, was also expressed in all cases. These results suggest that stromal cells also have vascular progenitor cell properties. In conclusion, stromal cells of hemangioblastomas exhibit mesenchymal stem cell-derived vascular progenitor cell properties.




Similar content being viewed by others
References
Rachinger J, Buslei R, Prell J et al (2009) Solid haemangioblastomas of the CNS: a review of 17 consecutive cases. Neurosurg Rev 32:37–47 (discussion 47–48)
Merrill MJ, Edwards NA, Lonser RR (2011) Notch receptor and effector expression in von Hippel-Lindau disease-associated central nervous system hemangioblastomas. J Neurosurg 115:512–517
Hussein MR (2007) Central nervous system capillary haemangioblastoma: the pathologist’s viewpoint. Int J Exp Pathol 88:311–324
Bamps S, Calenbergh FV, Vleeschouwer SD et al (2013) What the neurosurgeon should know about hemangioblastoma, both sporadic and in Von Hippel-Lindau disease: a literature review. Surg Neurol Int 4:145
Hojo M, Arakawa Y, Funaki T et al (2014) Usefulness of tumor blood flow imaging by intraoperative indocyanine green videoangiography in hemangioblastoma surgery. World Neurosurg 82:e495–e501
Spence AM, Rubinstein LJ (1975) Cerebellar capillary hemangioblastoma: its histogenesis studied by organ culture and electron microscopy. Cancer 35:326–341
Ding XH, Zhou LF, Tan YZ et al (2007) Histologic and histogenetic investigations of intracranial hemangioblastomas. Surg Neurol 67:239–245 (discussion 245)
Lach B, Gregor A, Rippstein P et al (1999) Angiogenic histogenesis of stromal cells in hemangioblastoma: ultrastructural and immunohistochemical study. Ultrastruct Pathol 23:299–310
Ishizawa K, Komori T, Hirose T (2005) Stromal cells in hemangioblastoma: neuroectodermal differentiation and morphological similarities to ependymoma. Pathol Int 55:377–385
Ma D, Zhang M, Chen L et al (2011) Hemangioblastomas might derive from neoplastic transformation of neural stem cells/progenitors in the specific niche. Carcinogenesis 32:102–109
Ma D, Zhu W, Zhang M et al (2011) Identification of tumorigenic cells and implication of their aberrant differentiation in human hemangioblastomas. Cancer Biol Ther 12:727–736
Park DM, Zhuang Z, Chen L et al (2007) von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 4:e60
Epari S, Bhatkar R, Moyaidi A et al (2014) Histomorphological spectrum and immunohistochemical characterization of hemangioblastomas: an entity of unclear histogenesis. Indian J Pathol Microbiol 57:542–548
Shively SB, Beltaifa S, Gehrs B et al (2008) Protracted haemangioblastic proliferation and differentiation in von Hippel-Lindau disease. J Pathol 216:514–520
Stein AA, Schilp AO, Whitfield RD (1960) The histogenesis of hemangioblastoma of the brain. A review of twenty-one cases. J Neurosurg 17:751–761
Welten CM, Keats EC, Ang LC et al (2012) Hemangioblastoma stromal cells show committed stem cell phenotype. Can J Neurol Sci 39:821–827
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Reyes M, Dudek A, Jahagirdar B et al (2002) Origin of endothelial progenitors in human postnatal bone marrow. J Clin Investig 109:337–346
Zhang G, Zhou J, Fan Q et al (2008) Arterial-venous endothelial cell fate is related to vascular endothelial growth factor and Notch status during human bone mesenchymal stem cell differentiation. FEBS Lett 582:2957–2964
Liu Y, Deng B, Zhao Y et al (2013) Differentiated markers in undifferentiated cells: expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells. Dev Growth Differ 55:591–605
Lin CH, Lilly B (2014) Endothelial cells direct mesenchymal stem cells toward a smooth muscle cell fate. Stem Cells Dev 23:2581–2590
Duffy GP, D’Arcy S, Ahsan T et al (2010) Mesenchymal stem cells overexpressing ephrin-b2 rapidly adopt an early endothelial phenotype with simultaneous reduction of osteogenic potential. Tissue Eng Part A 16:2755–2768
Kurpinski K, Lam H, Chu J et al (2010) Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28:734–742
Foo SS, Turner CJ, Adams S et al (2006) Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124:161–173
Wang HU, Chen ZF, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741–753
Adams RH, Wilkinson GA, Weiss C et al (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13:295–306
Hashimoto T, Tsuneki M, Foster TR et al (2016) Membrane-mediated regulation of vascular identity. Birth Defects Res C Embryo Today 108:65–84
Fischer A, Schumacher N, Maier M et al (2004) The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18:901–911
Fischer A, Gessler M (2003) Hey genes in cardiovascular development. Trends Cardiovasc Med 13:221–226
Kageyama R, Ohtsuka T, Shimojo H et al (2008) Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci 11:1247–1251
Lawson ND, Scheer N, Pham VN et al (2001) Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128:3675–3683
Hofmann JJ, Iruela-Arispe ML (2007) Notch signaling in blood vessels: who is talking to whom about what? Circ Res 100:1556–1568
Villa N, Walker L, Lindsell CE et al (2001) Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108:161–164
Roca C, Adams RH (2007) Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21:2511–2524
Swift MR, Weinstein BM (2009) Arterial-venous specification during development. Circ Res 104:576–588
Lonser RR, Glenn GM, Walther M et al (2003) von Hippel-Lindau disease. The Lancet 361:2059–2067
Takada S, Hojo M, Tanigaki K et al (2017) Contribution of endothelial-to-mesenchymal transition to the pathogenesis of human cerebral and orbital cavernous malformations. Neurosurgery 81:176–183
Takada S, Hojo M, Takebe N et al (2018) Role of endothelial-to-mesenchymal transition in the pathogenesis of central nervous system hemangioblastomas. World Neurosurg. https://doi.org/10.1016/j.wneu.2018.05.235 (in press)
Aruffo A, Stamenkovic I, Melnick M et al (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303–1313
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45
Oswald J, Boxberger S, Jrgensen B et al (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22:377–384
Boxall SA, Jones E (2012) Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012:975871
Böhling T, Mäenpää A, Timonen T et al (1996) Different expression of adhesion molecules on stromal cells and endothelial cells of capillary hemangioblastoma. Acta Neuropathol 92:461–466
Bai J, Wang YJ, Liu L et al (2014) Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J Int Med Res 42:405–415
Erber R, Eichelsbacher U, Powajbo V et al (2006) EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J 25:628–641
Hill-Felberg S, Wu HH, Toms SA et al (2015) Notch receptor expression in human brain arteriovenous malformations. J Cell Mol Med 19:1986–1993
Boucher J, Gridley T, Liaw L (2012) Molecular pathways of notch signaling in vascular smooth muscle cells. Front Physiol 3:81
Li S, Wang R, Wang Y et al (2014) Receptors of the Notch signaling pathway are associated with hemorrhage of brain arteriovenous malformations. Mol Med Rep 9:2233–2238
Yamashita J, Itoh H, Hirashima M et al (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408:92–96
Boscolo E, Stewart CL, Greenberger S et al (2011) JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31:2181–2192
Alles JU, Bosslet K, Schachenmayr W (1986) Hemangioblastoma of the cerebellum—an immunocytochemical study. Clin Neuropathol 5:238–241
Jurco SR, Nadji M, Harvey DG et al (1982) Hemangioblastomas: histogenesis of the stromal cell studied by immunocytochemistry. Hum Pathol 13:13–18
Mizuno J, Iwata K, Takei Y (1993) Immunohistochemical study of hemangioblastoma with special reference to its cytogenesis. Neurol Med Chir (Tokyo) 33:420–424
Bleistein M, Geiger K, Franz K et al (2000) Transthyretin and transferrin in hemangioblastoma stromal cells. Pathol Res Pract 196:675–681
Becker I, Paulus W, Roggendorf W (1989) Histogenesis of stromal cells in cerebellar hemangioblastomas. An immunohistochemical study. Am J Pathol 134:271–275
Nemes Z (1992) Fibrohistiocytic differentiation in capillary hemangioblastoma. Hum Pathol 23:805–810
Adams SA, Hilton DA (2002) Recurrent haemangioblastoma with glial differentiation. Neuropathol Appl Neurobiol 28:142–146
Tanimura A, Nakamura Y, Hachisuka H et al (1984) Hemangioblastoma of the central nervous system: nature of the stromal cells as studied by the immunoperoxidase technique. Hum Pathol 15:866–869
Gläsker S, Li J, Xia JB et al (2006) Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res 66:4167–4172
Shively SB, Falke EA, Li J et al (2011) Developmentally arrested structures preceding cerebellar tumors in von Hippel-Lindau disease. Mod Pathol 24:1023–1030
Vortmeyer AO, Frank S, Jeong SY et al (2003) Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res 63:7051–7055
Venkatesh V, Nataraj R, Thangaraj GS et al (2018) Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig 5:5
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (16689024 to Masato Hojo). We thank Ryota Ito, Mafumi Kurozumi and Toshiaki Manabe for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takada, S., Hojo, M., Takebe, N. et al. Stromal cells of hemangioblastomas exhibit mesenchymal stem cell-derived vascular progenitor cell properties. Brain Tumor Pathol 35, 193–201 (2018). https://doi.org/10.1007/s10014-018-0323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-018-0323-2