Abstract
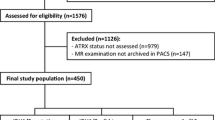
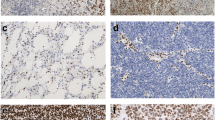
Recent studies on gliomas have shown frequent alterations in the alpha-thalassemia/mental retardation syndrome X-linked gene (ATRX). This study was designed to determine whether ATRX status correlates with uptake of 11C-methionine in WHO grades II and III gliomas. Sixty-two patients underwent 11C-methionine positron emission tomography scans prior to histological diagnosis. The tumor-to-normal ratio (T/N) of 11C-methionine uptake was calculated by dividing the maximum standardized uptake value (SUV) for the tumor by the mean SUV of the normal brain. After surgery, tumor samples were subjected to immunohistochemistry for ATRX and IDH1-R132H followed by IDH1/2 sequencing. Twenty-seven of the sixty-two patients were found to have the IDH mutation. Nine of the twenty-seven gliomas harboring IDH mutations exhibited loss of nuclear ATRX expression, which is accompanied with an astrocytic tumor lineage and a poor prognosis. The mean T/N ratio in tumors with loss of nuclear ATRX expression was 2.20 ± 0.53, i.e., significantly lower than that of tumors with ATRX retention (3.28 ± 1.32, p = 0.0171, U test). Our study showed ATRX status to correlate with the T/N ratio and the outcomes of WHO grade II and III glioma patients with the IDH1 mutation. Our data provide new information on the biology and imaging characteristics of gliomas.




Similar content being viewed by others
References
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Suzuki H, Aoki K, Chiba K et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47:458–468
Liu XY, Gerges N, Korshunov A et al (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124:615–625
Jiao Y, Killela PJ, Reitman ZJ et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Saito T, Maruyama T, Muragaki Y et al (2013) 11 C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am J Neuroradiol 34:85–91
Iwadate Y, Shinozaki N, Matsutani T et al (2016) Molecular imaging of 1p/19q deletion in oligodendroglial tumours with 11 C-methionine positron emission tomography. J Neurol Neurosurg Psychiatry 87:1016–1021
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474
Wiestler B, Capper D, Holland-Letz T et al (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451
Reuss DE, Sahm F, Schrimpf D et al (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 129:133–146
Nojiri T, Nariai T, Aoyagi M et al (2009) Contributions of biological tumor parameters to the incorporation rate of L: -[methyl-(11)C] methionine into astrocytomas and oligodendrogliomas. J Neurooncol 93:233–241
Nariai T, Tanaka Y, Wakimoto H et al (2005) Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 103:498–507
Shinozaki N, Uchino Y, Yoshikawa K et al (2011) Discrimination between low-grade oligodendrogliomas and diffuse astrocytoma with the aid of 11 C-methionine positron emission tomography. J Neurosurg 114:1640–1647
Louis DN, Ohgaki H, Wiestler OD et al (2007) World Health Organization histological classi cation of tumours of the central nervous system. International Agency for Research on Cancer, Lyon
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Ishiwata K, Kubota K, Murakami M et al (1993) Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med 34:1936–1943
Langen KJ, Muhlensiepen H, Holschbach M et al (2000) Transport mechanisms of 3-[123I]iodo-alpha-methyl-l-tyrosine in a human glioma cell line: comparison with [3H]methyl]-l-methionine. J Nucl Med 41:1250–1255
Bergstrom M, Lundqvist H, Ericson K et al (1987) Comparison of the accumulation kinetics of l-(methyl-11C)-methionine and d-(methyl-11C)-methionine in brain tumors studied with positron emission tomography. Acta Radiol 28:225–229
Wienhard K, Herholz K, Coenen HH et al (1991) Increased amino acid transport into brain tumors measured by PET of L-(2-18F)fluorotyrosine. J Nucl Med 32:1338–1346
Cha S, Tihan T, Crawford F et al (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 26:266–273
Lee JC, Jeng YM, Liau JY et al (2015) Alternative lengthening of telomeres and loss of ATRX are frequent events in pleomorphic and dedifferentiated liposarcomas. Mod Pathol 28:1064–1073
Marinoni I, Kurrer AS, Vassella E et al (2014) Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 146(453–460):e455
Kannan K, Inagaki A, Silber J et al (2012) Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3:1194–1203
Ikemura M, Shibahara J, Mukasa A et al (2016) Utility of ATRX immunohistochemistry in diagnosis of adult diffuse gliomas. Histopathology 69:260–267
Acknowledgements
We thank Dr. Hiroaki Wakimoto (Harvard University, Boston) for invaluable advice. This work was supported by JSPS KAKENHI Grant Number 15K19954 (K. Tamura). Portions of this study were presented in abstract form at the 34th Annual Meeting of the Japan Society of Brain Tumor Pathology, Tokyo, Japan, May 27, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
This study was approved by the Ethics Committee of the Tokyo Medical and Dental University. Informed consent was obtained from the patients and/or their guardians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogishima, T., Tamura, K., Kobayashi, D. et al. ATRX status correlates with 11 C-methionine uptake in WHO grade II and III gliomas with IDH1 mutations. Brain Tumor Pathol 34, 20–27 (2017). https://doi.org/10.1007/s10014-017-0280-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-017-0280-1