Abstract
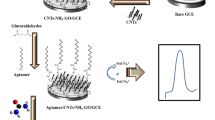
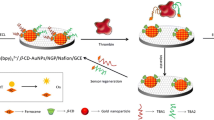
A simple and sensitive bifunctional electrochemical aptasensor for detection of adenosine and thrombin has been developed using gold nanoparticles–electrochemically reduced graphene oxide (AuNPs-ERGO) composite film-modified electrode. Firstly, the reduced graphene oxide film and AuNPs were sequentially immobilized on glassy carbon electrode (GCE) surface. Secondly, thrombin aptamer was immobilized on the modified electrode. Finally, adenosine aptamer was hybridized with it to serve as a recognition element and methylene blue (MB) as electrochemical signal indicator. In the presence of adenosine or thrombin, the sensor recognized it and a conformational change was induced in aptamer, resulting in decrease of the peak current of MB. The linear relation between concentration of adenosine or thrombin and peak current of MB allowed quantification of them. Thanks to the special electronic characteristic of AuNPs-ERGO composite film, sensitivity of sensor was greatly improved. Under optimal conditions, the proposed aptasensor presented an excellent performance in a linear range of 25 nM to 750 nM for adenosine and 0.5 nM to 10 nM for thrombin. Detection limits were estimated to be 8.3 nM for adenosine and 0.17 nM for thrombin, respectively. Moreover, dual-analyte detection of adenosine and thrombin was achieved without potentially increasing the complexity and cost of the assay.







Similar content being viewed by others
References
Zhang D, Ma JJ, Meng XW, Xu ZF, Zhang J, Fang YX, Guo Y (2019) Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal Chim Acta 1076:55–63
Urbanová V, Jayaramulu K, Schneemann A, Kment Š, Fischer RA, Zbořil R (2018) Hierarchical porous fluorinated graphene oxide@metal-organic gel composite: label-free electrochemical aptasensor for selective detection of thrombin. ACS Appl Mater Interfaces 10(48):41089–41097
Xu YY, Zhou WJ, Zhou M, Xiang Y, Yuan R, Chai YQ (2015) Toehold strand displacement-driven assembly of G-quadruplex DNA for enzyme-free and non-label sensitive fluorescent detection of thrombin. Biosens Bioelectron 64:306–310
Chen ZB, Tan Y, Zhang CM, Yin L, Ma H, Ye NS, Qiang H, Lin YQ (2014) A colorimetric aptamer biosensor based on cationic polymer and gold nanoparticles for the ultrasensitive detection of thrombin. Biosens Bioelectron 56:46–50
Man TT, Lai W, Xiao MS, Wang XW, Chandrasekaran AR, Pei H, Li L (2020) A versatile biomolecular detection platform based on photo-induced enhanced Raman spectroscopy. Biosens Bioelectron 147:111742
Zhu Y, Hu XC, Shi S, Gao RR, Huang HL, Zhu YY, Lv XY, Yao TM (2016) Ultrasensitive and universal fluorescent aptasensor for the detection of biomolecules (ATP, adenosine and thrombin) based on DNA/Ag nanoclusters fluorescence light-up system. Biosens Bioelectron 79:205–212
Zheng J, Li NX, Li CR, Wang XX, Liu YC, Mao GB, Ji XH, He ZK (2018) A nonenzymatic DNA nanomachine for biomolecular detection by target recycling of hairpin DNA cascade amplification. Biosens Bioelectron 107:40–46
Zhang SS, Xia JP, Li XM (2008) Electrochemical biosensor for detection of adenosine based on structure-switching aptamer and amplification with reporter probe DNA modified Au nanoparticles. Anal Chem 80(22):8382–8388
Jalil O, Pandey CM, Kumar D (2020) Electrochemical biosensor for the epithelial cancer biomarker EpCAM based on reduced graphene oxide modified with nanostructured titanium dioxide. Microchim Acta 187(5):275
Zhou ZD, Gu CM, Chen C, Zhao PC, Xie YX, Fei JJ (2019) An ultrasensitive electrochemical sensor for quercetin based on 1-pyrenebutyrate functionalized reduced oxide graphene/mercapto-β-cyclodextrin/Au nanoparticles composite film. Sensors Actuators B Chem 288:88–95
Hanko M, Švorc L, Planková A, Mikuš P (2019) Overview and recent advances in electrochemical sensing of glutathione-a review. Anal Chim Acta 1062:1–27
Haque AMJ, Park H, Sung D, Jon S, Choi SY, Kim K (2012) An electrochemically reduced graphene oxide-based electrochemical immunosensing platform for ultrasensitive antigen detection. Anal Chem 84(4):1871–1878
Shamsipur M, Farzin L, Tabrizi MA (2016) Ultrasensitive aptamer-based on-off assay for lysozyme using a glassy carbon electrode modified with gold nanoparticles and electrochemically reduced graphene oxide. Microchim Acta 183(10):2733–2743
Vinoth V, Wu JJ, Asiri AM, Anandan S (2017) Sonochemical synthesis of silver nanoparticles anchored reduced graphene oxide nanosheets for selective and sensitive detection of glutathione. Ultrason Sonochem 39:363–373
Fan LF, Zhang CY, Yan WJ, Guo YJ, Shuang SM, Dong C, Bi YP (2019) Design of a facile and label-free electrochemical aptasensor for detection of atrazine. Talanta 201:156–164
Ye YK, Yan WW, Liu YQ, He SD, Cao XD, Xu X, Zheng HS, Gunasekaran S (2019) Electrochemical detection of Salmonella using an invA genosensor on polypyrrole-reduced graphene oxide modified glassy carbon electrode and AuNPs-horseradish peroxidase-streptavidin as nanotag. Anal Chim Acta 1074:80–88
Yang SH, Zhang FF, Liang QL, Wang ZH (2018) A three-dimensional graphene-based ratiometric signal amplification aptasensor for MUC1 detection. Biosens Bioelectron 120:85–92
Yang JM, Li XL, Jiang BY, Yuan R, Xiang Y (2020) In situ-generated multivalent aptamer network for efficient capture and sensitive electrochemical detection of circulating tumor cells in whole blood. Anal Chem 92(11):7893–7899
Zhang LL, Zhang XY, Feng PJ, Han Q, Liu W, Lu Y, Song CX, Li FY (2020) Photodriven regeneration of G-Quadruplex aptasensor for sensitively detecting thrombin. Anal Chem 92(11):7419–7424
Qi LJ, Han X, Du Y (2020) Improved sensitivity for ratiometric fluorescence detection of ricin based on “kinetic competition” aptasensing strategy. Sensors Actuators B Chem 314:128073
Xiang WW, Lv QX, Shi HX, Xie B, Gao L (2020) Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 214:120716
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
So HM, Won K, Kim YH, Kim BK, Ryu BH, Na PS, Kim H, Lee JO (2005) Single-walled carbon nanotube biosensors using aptamers as molecular recognition elements. J Am Chem Soc 127(34):11906–11907
Liu JW, Lu Y (2006) Fast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles. Angew Chem Int Ed 45(1):90–94
Centi S, Tombelli S, Minunni M, Mascini M (2007) Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem 79(4):1466–1473
Zhu C, Li LS, Wang ZJ, Irfan M, Qu F (2020) Recent advances of aptasensors for exosomes detection. Biosens Bioelectron 160:112213
Ding LH, Wu YJ, Liu W, Liu L, Yu F, Yu SC, Tian YM, Feng JD, He LL (2019) Magnetic-assisted self-assembled aptamer/protein hybrid probes for efficient capture and rapid detection of cancer cells in whole blood. Talanta 205:120129
Xu YX, Bai H, Lu GW, Li C, Shi GQ (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130(18):5856–5857
Shan CS, Yang HF, Song JF, Han DX, Ivaska A, Niu L (2009) Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem 81(6):2378–2382
Zhu XH, Zeng LX, Xu MT, Liang Y, Nan JM (2012) A glassy carbon electrode modified with electrochemically reduced graphene for simultaneous determination of guanine and adenine. Anal Methods 4(9):2935–2939
Dong YP, Zhou Y, Wang J, Zhu JJ (2016) Electrogenerated chemiluminescence resonance energy transfer between Ru(bpy)32+ electrogenerated chemiluminescence and gold nanoparticles/graphene oxide nanocomposites with graphene oxide as coreactant and its sensing application. Anal Chem 88(10):5469–5475
Edris NMMA, Abdullah J, Kamaruzaman S, Saiman MI, Sulaiman Y (2018) Electrochemical reduced graphene oxide-poly(eriochrome black T)/gold nanoparticles modified glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Arab J Chem 11(8):1301–1312
Yang SL, Lu ZZ, Luo SL, Liu CB, Tang YH (2013) Direct electrodeposition of a biocomposite consisting of reduced graphene oxide, chitosan and glucose oxidase on a glassy carbon electrode for direct sensing of glucose. Microchim Acta 180(1-2):127–135
Wang J, Li BZ, Lu QY, Li XY, Weng CY, Yan XQ, Hong JL, Zhou XM (2019) A versatile fluorometric aptasensing scheme based on the use of a hybrid material composed of polypyrrole nanoparticles and DNA-silver nanoclusters: application to the determination of adenosine, thrombin, or interferon-gamma. Microchim Acta 186(6):356
Feng CJ, Hou Z, Jiang W, Sang LH, Wang L (2016) Binding induced colocalization activated hybridization chain reaction on the surface of magnetic nanobead for sensitive detection of adenosine. Biosens Bioelectron 86:966–970
Sun JW, Jiang W, Zhu J, Li W, Wang L (2015) Label-free fluorescence dual-amplified detection of adenosine based on exonuclease III-assisted DNA cycling and hybridization chain reaction. Biosens Bioelectron 70:15–20
Xu SC, Man BY, Jiang SZ, Wang JH, Wei J, Xu SD, Liu HP, Gao SB, Liu HL, Li ZH, Li HS, Qiu HW (2015) Graphene/Cu nanoparticle hybrids fabricated by chemical vapor deposition as surface-enhanced Raman scattering substrate for label-free detection of adenosine. ACS Appl Mater Interfaces 7(20):10977–10987
Xu SC, Jiang SZ, Wang JH, Wei J, Yue WW, Ma Y (2016) Graphene isolated Au nanoparticle arrays with high reproducibility for high-performance surface-enhanced Raman scattering. Sensors Actuators B Chem 222:1175–1183
Sun QQ, Yan F, Su B (2018) Label-free electrochemical biosensors based on 3,3′,5,5′-tetramethylbenzidine responsive isoporous silica-micelle membrane. Biosens Bioelectron 105:129–136
Li F, Du ZF, Yang LM, Tang B (2013) Selective and sensitive turn-on detection of adenosine triphosphate and thrombin based on bifunctional fluorescent oligonucleotide probe. Biosens Bioelectron 41:907–910
Gao BB, Tang LTY, Zhang DG, Xie ZY, Su EB, Liu H, Gu ZZ (2017) Transpiration-inspired fabrication of opal capillary with multiple heterostructures for multiplex aptamer-based fluorescent assays. ACS Appl Mater Interfaces 9(38):32577–32582
Du F, Alam MN, Pawliszyn J (2014) Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal Chim Acta 845:45–52
Funding
This study is supported by the National Natural Science Foundation of China (21778041).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, H., Hu, P., Tang, J. et al. A bifunctional electrochemical aptasensor based on AuNPs-coated ERGO nanosheets for sensitive detection of adenosine and thrombin. J Solid State Electrochem 25, 1383–1391 (2021). https://doi.org/10.1007/s10008-021-04916-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04916-y