Abstract
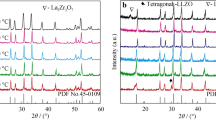
Ga-doped Li7La3Zr2O12 (Ga-LLZO) is a promising solid electrolyte because it shows higher Li-ion conductivity than LLZO doped with other cations. In this work, Ga-LLZO was prepared by a conventional solid-state reaction and sintered in ZrO2 crucibles to avoid introducing Al into the samples. The particle size distribution, phase structure, morphology, ionic conductivity, and bulk density of the samples were characterized by laser diffraction particle size analyzer, X-ray diffraction (XRD), scanning electron microscope (SEM), AC impedance, and Archimedes method, respectively. The effects of sintering temperature and dopant content on Li-ion conductivity and density were investigated. As for Al-free Li6.4Ga0.2La3Zr2O12, the Li-ion conductivity and bulk density are improved with the sintering temperature rise. But when the sintering temperature is 1250 °C, the Li-ion conductivity and bulk density decline. As for Al-free Li7-3xGaxLa3Zr2O12, the tetragonal phase disappears completely when x ≥ 0.15. The Li-ion conductivity and bulk density of Al-free Li7-3xGaxLa3Zr2O12 are improved with an increase of x (x ≤ 0.2). The ionic conductivity of Al-free Li7-3xGaxLa3Zr2O12 sintered in the air by liquid-phase sintering is close to Ga-LLZO sintered in dry O2 atmosphere. Li6.4Ga0.2La3Zr2O12 sintered at 1200 °C shows the highest Li-ion conductivity of 1.5 × 10−3 S/cm at 30 °C, and the activation energy is about 0.28 eV.





Similar content being viewed by others
References
Wu JF, Pang WK, Peterson VK, Wei L, Guo X (2017) Garnet-type fast Li-ion conductors with high ionic conductivities for all-solid-state batteries. ACS Appl Mater Interfaces 9:12461–12468
Thangadurai V, Narayanan S, Pinzaru D (2014) Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem Soc Rev 43:4714–4727
Jin Y, McGinn PJ (2013) Li7La3Zr2O12 electrolyte stability in air and fabrication of a Li/ Li7La3Zr2O12 /Cu0.1V2O5 solid-state battery. J Power Sources 239:326–331
Wang XP, Xia Y, Hu J, Xia YP, Zhuang Z, Guo LJ, Lu H, Zhang T, Fang QF (2013) Phase transition and conductivity improvement of tetragonal fast lithium ionic electrolyte Li7La3Zr2O12. Solid State Ionics 253:137–142
Cussen EJ (2010) Structure and ionic conductivity in lithium garnets. J Mater Chem 20:5167–5173
Rosenkiewitz N, Schuhmacher J, Bockmeyer M, Deubener J (2015) Nitrogen-free sol-gel synthesis of Al-substituted cubic garnet Li7La3Zr2O12 (LLZO). J Power Sources 278:104–108
Wagner R, Redhammer GJ, Rettenwander D, Tippelt G, Welzl A, Taibl S, Fleig J, Franz A, Lottermoser W, Amthauer G (2016) Fast Li-ion-conducting garnet-related Li7–3xFexLa3Zr2O12 with uncommon I-43d structure. Chem Mater 28:5943–5951
Awaka J, Kijima N, Hayakawa H, Akimoto J (2009) Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J Solid State Chem 182:2046–2052
Awaka J, Kijima N, Kataoka K, Hayakawa H, Ohshima K, Akimoto J (2010) Neutron powder diffraction study of tetragonal Li7La3Hf2O12 with the garnet-related type structure. J Solid State Chem 183:180–185
Awaka J, Takashima A, Kataoka K, Kijima N, Idemoto Y, Akimoto J (2011) Crystal structure of fast lithium-ion-conducting cubic Li7La3Zr2O12. Chem Lett 40:60–62
Ramakumar S, Deviannapoorani C, Dhivya L, Shanker LS, Murugan R (2017) Lithium garnets: synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci 88:325–411
Cheng L, Wu CH, Jarry A, Chen W, Ye Y, Zhu J, Kostecki R, Persson K, Guo J, Salmeron M, Chen G, Doeff M (2015) Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12. ACS Appl Mater Interfaces 7:17649–17655
Cheng L, Park JS, Hou H, Zorba V, Chen G, Richardson T, Cabana J, Russo R, Doeff M (2014) Effect of microstructure and surface impurity segregation on the electrical and electrochemical properties of dense Al-substituted Li7La3Zr2O12. J Mater Chem A 2:172–181
Jin Y, McGinn PJ (2011) Al-doped Li7La3Zr2O12, synthesized by a polymerized complex method. J Power Sources 196:8683–8687
Janani N, Deviannapoorani C, Dhivya L, Murugan R (2014) Influence of sintering additives on densification and Li+ conductivity of Al doped Li7La3Zr2O12 lithium garnet. RSC Adv 4:51228–51238
Buannic L, Orayech B, López Del Amo J-M, Carrasco J, Katcho NA, Aguesse F, Manalastas W, Zhang W, Kilner J, Llordés A (2017) Dual substitution strategy to enhance Li+ ionic conductivity in Li7La3Zr2O12 solid electrolyte. Chem Mater 29:1769–1778
Matsui M, Takahashi K, Sakamoto K, Hirano A, Takeda Y, Yamamoto O, Imanishi N (2014) Phase stability of a garnet-type lithium ion conductor Li7La3Zr2O12. Dalton Trans 43:1019–1024
Jalem R, Rushton MJD, Manalastas William J, Nakayama M, Kasuga T, Kilner JA, Grimes RW (2015) Effects of gallium doping in garnet-type Li7La3Zr2O12 solid electrolytes. Chem Mater 27:2821–2831
Thompson T, Wolfenstine J, Allen JL, Johannes M, Huq A, David IN, Sakamoto J (2014) Tetragonal vs. cubic phase stability in Al-free Ta doped Li7La3Zr2O12 (LLZO). J Mater Chem A 2:13431–13436
Zhang Y, Chen F, Tu R, Shen Q, Zhang L (2014) Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. J Power Sources 268:960–964
Shinawi HE, Janek J (2013) Stabilization of cubic lithium-stuffed garnets of the type “Li7La3Zr2O12” by addition of gallium. J Power Sources 225:13–19
Rettenwander D, Geiger CA, Tribus M, Tropper P, Amthauer GA (2014) Synthesis and crystal chemical study of the fast ion conductor Li7−3xGaxLa3Zr2O12 with x = 0.08 to 0.84. Inorg Chem 53:6264–6269
Rettenwander D, Redhammer G, Preishuber-Pflügl F, Cheng L, Miara L, Wagner R, Welzl A, Suard E, Doeff MM, Wilkening M, Fleig J, Amthauer G (2016) Structural and electrochemical consequences of Al and Ga cosubstitution in Li7La3Zr2O12 solid electrolytes. Chem Mater 28:2384–2392
Wagner R, Redhammer GJ, Rettenwander D, Senyshyn A, Schmidt W, Wilkening M, Amthauer G (2016) Crystal structure of garnet-related Li-ion conductor Li7−3xGaxLa3Zr2O12: fast Li-ion conduction caused by a different cubic modification? Chem Mater 28:1861–1871
Bernuy-Lopez C, Manalastas W, Amo JMLD, Aguadero A, Aguesse F, Kilner JA (2014) Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics. Chem Mater 26:3610–3617
Li Y, Wang Z, Li C, Cao Y, Guo X (2014) Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J Power Sources 248:642–646
Li Y, Han J-T, Vogel S, Wang C-A (2015) The reaction of Li6.5La3Zr1.5 Ta0.5O12 with water. Solid State Ionics 269:57–61
Matsuda Y, Sakaida A, Sugimoto K, Mori D, Takeda Y, Yamamoto O, Imanishi N (2017) Sintering behavior and electrochemical properties of garnet-like lithium conductor Li6.25M0.25La3Zr2O12 (M: Al3+ and Ga3+). Solid State Ionics 311:69–74
German RM, Suri P, Park SJ (2009) Review: liquid phase sintering. J Mater Sci 44:1–39
Wu J-F, Chen E-Y, Yu Y, Liu L, Wu Y, Pang W, Peterson V, Guo X (2017) Gallium-doped Li7La3Zr2O12 garnet-type electrolytes with high lithium-ion conductivity. Appl Mater Interfaces 9:1542–1155
Funding
The authors acknowledge the financial support from the National Natural Science Foundation of China (51374055).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yi, M., Liu, T., Li, J. et al. High Li-ion conductivity of Al-free Li7-3xGaxLa3Zr2O12 solid electrolyte prepared by liquid-phase sintering. J Solid State Electrochem 23, 1249–1256 (2019). https://doi.org/10.1007/s10008-019-04225-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04225-5