Abstract
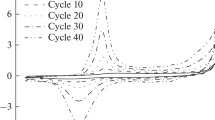
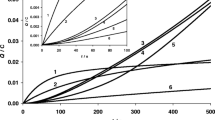
Polyaniline (PANI) represents itself a class of polymers having ionic and electronic conductivities. The polymer has wide application as chemical modifier of electrodes for voltammetry. Electrochemical polymerization of aniline includes in itself some oxidation/reduction stages of electroactive groups. In the result of this process, polyaniline structures of different contents are formed. The qualitative and quantitative contents of polymer film and its area and morphology during the polymerization process play an important role in electroconductivity and response of the final synthesis product on analyte. We demonstrate a possibility of quantitative description of the polyaniline electrochemical polymerization process in the frame of percolation model covering wide temporal range (100–500 reduction/oxidation cycles). We can evaluate the basic parameters (number of percolation channels and fractal dimensions with respect to the applied potential) characterizing the quality of electrode polymer cover. The microphotos obtained with the help of the high-resolution scanning electron microscope support the obtained results independently.

















Similar content being viewed by others
Abbreviations
- DEL:
-
Double electric layer
- DLA:
-
Diffusion-limited aggregation
- SEM:
-
Scanning electron microscope.
- FD:
-
Fractal dimension
- FLSM:
-
Functional linear square method
- IM:
-
Intermediate model
- LLSM:
-
Linear least square method
- PANI:
-
Polyaniline
- PM:
-
Percolation model
- VAG(s):
-
Voltammogram(s)
References
Budnikov H, Shirokova V (2013) Term “nano” in electroanalysis: a trendy prefix or a new stage of its development? J Anal Chem 68(8):663–670
Nigmatullin R, Budnikov H, Sidelnikov A (2018) Mesoscopic theory of percolation currents associated with quantitative description of VAGs: confirmation on real data. Chaos, Solitons Fractals 106:171–183
Diab N, AbuZuhri A, Schuhmann W (2003) Sequential-injection stripping analysis of nifuroxime using DNA-modified glassy carbon electrodes. Bioelectrochemistry 61(1-2):57–63
Moss R, Pérez-Roa R, Anderson M (2013) Electrochemical response of titania, zirconia, and alumina electrodes to phosphate adsorption. Electrochim Acta 104:314–321
Battistel D, Daniele S (2013) Determination of trace bismuth by under-potential deposition-stripping voltammetry at mesoporous platinum microelectrodes: application to pharmaceutical products. J Solid State Electrochem 17(6):1509–1516
Wang J, Kawde A (2001) Pencil-based renewable biosensor for label-free electrochemical detection of DNA hybridization. Anal Chim Acta 431(2):219–224
Biallozor S, Kupniewska A (2005) Conducting polymers electrodeposited on active metals. Synth Met 155(3):443–449
Mu S, Yang Y (2008) Spectral characteristics of polyaniline nanostructures synthesized by using cyclic voltammetry at different scan rates. J Phys Chem B 112(37):11558–11563
Gvozdenović M, Jugović B, Stevanović J, Grgur B (2014) Electrochemical synthesis of electroconducting polymers. Hem Ind 68(6):673–684
Nigmatullin R, Sidelnikov A, Budnikov H, Maksyutova E (2018) Description of complex fluids electrochemical data in the frame of percolation model. Electroanal 30(9):2053–2065
Abrantes LM, Correia JP, Savic M, Jin G (2001) Structural modifications during conducting polymer formation—an ellipsometric study. Electrochim Acta 46(20-21):3181–3187
Rego LS, Antonio JL, Silva CH, Nobrega MM, Temperini ML, Torresi RM, de Torresi SIC (2016) Electrochemical template synthesis of adherent polyaniline thin films with tubular structure. J Solid State Electrochem 20(4):983–991
Wang J, Zhang K, Xu H, Yan B, Gao F, Shi Y, Du Y (2018) Engineered photoelectrochemical platform for the ultrasensitive detection of caffeic acid based on flower-like MoS2 and PANI nanotubes nanohybrid. Sensors Actuators B Chem 276:322–330
Liu X, Shang P, Zhang Y, Wang X, Fan Z, Wang B, Zheng Y (2014) Three-dimensional and stable polyaniline-grafted graphene hybrid materials for supercapacitor electrodes. J Mater Chem A 2(37):15273–15278
Liu X, Zheng Y, Wang X (2015) Controllable preparation of polyaniline–graphene nanocomposites using functionalized graphene for supercapacitor electrodes. Chem Eur J 21(29):10408–10415
Skotheim TA, Reynolds J (2006) Conjugated polymers: theory, synthesis, properties, and characterization. CRC Press, Boca Raton
Inzelt G (2012) Conducting polymers: a new era in electrochemistry. In: Scholz F (ed) Monographs in electrochemistry, 2nd edn. Springer, Berlin
Wallace G, Spinks G, Kane-Maguire L, Teasdale P (2009) Conductive electroactive polymers: intelligent polymer systems. CRC press, Boca Raton
Zotti G, Cattarin S, Comisso N (1987) Electrodeposition of polythiophene, polypyrrole and polyaniline by the cyclic potential sweep method. J Electroanal Chem 235(1-2):259–273
Hussain A, Kumar A (2003) Electrochemical synthesis and characterization of chloride doped polyaniline. Bull Mater Sci 26(3):329–334
Mohammad F (2001) Handbook of advanced electronic and photonic materials and devices. Academic Press, Cambridge
Pud A (1994) Stability and degradation of conducting polymers in electrochemical systems. Synth Met 66(1):1–18
Tarasevich M, Orlov S, Shkolnikov E (1990) Electrochemistry of polymers. Science, Moscow
Funding
This work was supported by the Russian Foundation Basic Research Project № 17-43-020232 r-Povolzh’ye-а.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nigmatullin, R.R., Budnikov, H.C., Mustafin, A.G. et al. Process of electrochemical electrode modification by polyaniline in the frame of percolation model. J Solid State Electrochem 23, 1221–1235 (2019). https://doi.org/10.1007/s10008-019-04217-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04217-5