Abstract
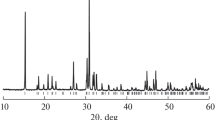
The Ca2.5Mg2V3O12 vanadate with cubic structure (space group Ia\( \overline{3} \)d) has been prepared by solid-state reaction in air. The crystal structure has been determined by Rietveld refinement of powder X-ray diffraction data. In the garnet-type structure, the Ca2+ ions and cationic vacancies occupy the 24c sites. Conductivity has been measured using AC impedance spectroscopy at 400–950 °С in air and in the range of oxygen partial pressure \( \left({p}_{{\mathrm{O}}_2}\right) \) between 10−10 and 0.5 atm. Ca2.5Mg2V3O12 exhibits mixed ionic and electronic conductivity. The n-type electronic conductivity is proportional to \( {p}_{{\mathrm{O}}_2}^{-1/4} \). The ionic and electronic components of conductivity are characterized by the activation energy of about 1.2 and 2.0 eV, respectively. Ion transference number in the air has been shown to increase from 0.6 to almost 1.0 upon temperature decrease from 950 to 600 °С. Analysis of the garnet-like crystal structure allows proposing possible mechanisms of ionic conductivity in which a Ca-ion migration pathway from occupied 24c sites to vacant 24c′ position is carried out through empty 16b sites.









Similar content being viewed by others
References
Yao GG, Liu P, Zhang HW (2013) Novel series of low-firing microwave dielectric ceramics: Ca5 A 4(VO4)6 (A 2+=Mg, Zn). J Am Ceram Soc 96(6):1691–1693
Yao G, Pei C, Ma H, Xu J, Liu P, Zhang H (2017) Low-temperature firing and microwave dielectric properties of (1–x)Ca5Mg4(VO4)6–xBa3(VO4)2 temperature stable ceramics. J Alloys Compd 709:234–239
Huang YL, Yu YM, Tsuboi TJ, Seo HJ (2012) Novel yellow-emitting phosphors of Ca5 M 4(VO4)6 (M=Mg, Zn) with isolated VO4 tetrahedra. Opt Express 20(4):4360–4368
Li K, Liu J, Mara D, Van Deun R (2018) Synthesis and up-conversion luminescence properties of a novel Yb3+, Er3+ co-doped Ca5Mg4(VO4)6 phosphor. J Alloys Compd 737:767–773
Ronniger G, Mill' BV (1973) Vanadates with a defect garnet structure. Sov Phys Crystallogr 18:303–307
Leonidov IA, Belik AA, Leonidova ON, Lazoryak BI (2002) Structural aspects of calcium ion transport in Ca3(VO4)2 and Ca3–xNd2x/3(VO4)2 solid solutions. Russ J Inorg Chem 47:305–312
Thangadurai V, Adams S, Weppner W (2004) Crystal structure revision and identification of Li+-ion migration pathways in the garnet-like Li5La3 M 2O12 (M = Nb, Ta) oxides. Chem Mater 16(16):2998–3006
Gu W, Ezbiri M, Prasada Rao R, Avdeev M, Adams S (2015) Effects of penta- and trivalent dopants on structure and conductivity of Li7La3Zr2O12. Solid State Ionics 274:100–105
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192(1-2):55–69
Patrakeev MV, Mitberg EB, Leonidov IA, Kozhevnikov VL (2001) Electrical characterization of the intergrowth ferrite Sr4Fe6O13+δ. Solid State Ionics 139(3-4):325–330
Iishi K, Ikuta Y (2006) Isomorphous substitutions in vanadate garnets. N Jb Miner 182:157–163
Li YT, Han JT, Wang CA, Vogel SC, Xie H, Xu MW, Goodenough JB (2012) Ionic distribution and conductivity in lithium garnet Li7La3Zr2O12. J Power Sources 209:278–281
Reddy MV, Adams S (2017) Molten salt synthesis and characterization of fast ion conductor Li6.75La3Zr1.75Ta0.25O12. J Solid State Electrochem 21(10):2921–2928
Leonidov IA, Leonidova ON, Slepukhin VK (2000) Electronic conductivity of Sr3–3xLa2x(VO4)2 solid solutions. Inorg Mater 36:72–75
Kofstad P (1972) Nonstoichiometry, diffusion and electrical conductivity in binary metal oxides. Wiley-Interscience, New York
Voronkova VI, Leonidov IA, Kharitonova EP, Belov DA, Patrakeev MV, Leonidova ON, Kozhevnikov VL (2014) Oxygen ion and electron conductivity in fluorite-like molybdates Nd5Mo3O16 and Pr5Mo3O16. J Alloys Compd 615:395–400
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Crystallogr Sect B 25(5):925–946
Leonidov IA, Leonidova ON, Surat LL, Samigullina RF (2003) Ca3(VO4)2–LaVO4 cation conductors. Inorg Mater 39(6):616–620
Funding
This work was partially supported by the Government Research Program for the Institute of Solid State Chemistry and UB RAS (Grant No. 18–10–3–32).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leonidova, O.N., Patrakeev, M.V. & Leonidov, I.A. Ionic and electronic transport in the garnet-type vanadate Ca2.5Mg2V3O12. J Solid State Electrochem 23, 1083–1088 (2019). https://doi.org/10.1007/s10008-019-04202-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04202-y