Abstract
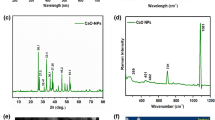
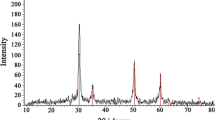
Cerium-doped zirconium oxide (Ce/ZrO2) was introduced as a highly efficient electrocatalyst for electrooxidation of salicylic acid (SA). The electrocatalyst material was synthesized via co-precipitation of cerium and zirconium ions, and then the resulting solid was heat-treated at high temperature to create crystallized cerium-doped zirconium oxide nanoparticles. The obtained material was characterized by scanning electron microscopy and X-ray diffraction methods. The Ce/ZrO2-modified carbon paste electrode (Ce/ZrO2-CPE) exhibited a distinct oxidative peak for SA, whereas no signal was observed for SA at unmodified carbon paste electrode at the same experimental conditions. Cyclic voltammetry and electrochemical impedance spectroscopy were applied to investigate the electrocatalytic performance of the electrode and SA electrooxidation mechanism. Square wave voltammetry was used to capture the analytical signal of SA. The electrode composition was optimized to increase the SA signal. Using the optimized electrode, it became possible to determine SA in the concentration range of 5.0–1000.0 μM with detection limit of 1.1 μM (3Sb/m). The electrode showed very high sensitivity of 1013.5 μA mM−1 cm−2 which is remarkably better than the previously reported SA sensors. The proposed method was successfully applied for the determination of SA in human serum, milk, and pharmaceutical samples.







Similar content being viewed by others
References
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43(1):439–463. https://doi.org/10.1146/annurev.pp.43.060192.002255
Wang LJ, Fan L, Loescher W, Duan W, Liu G, Cheng JS, Luo HB, Li SH (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10(1):34–39. https://doi.org/10.1186/1471-2229-10-34
Ehrendorfer M, Sontag G, Pittner F (1996) Determination of salicylate in beverages and cosmetics by use of an amperometric biosensor. Fresenius J Anal Chem 356(1):75–79. https://doi.org/10.1007/s0021663560075
Zhu Y, Guan X, Ji H (2009) Electrochemical solid phase micro-extraction and determination of salicylic acid from blood samples by cyclic voltammetry and differential pulse voltammetry. J Solid State Electrochem 13(9):1417–1423. https://doi.org/10.1007/s10008-008-0707-z
Chem BE, Johns D, Bochner F, Imhoff OM, Rowland M (1997) Simultaneous liquid-chromatographic quantitation of salicylic acid, salicyluric acid, and gentisic acid in plasma. Clin Chem 25:1420–1425
Rainsford KD, Aspirin and the salicylates: Butterworths, London U.K., 1984, pp.245–248
Saha U, Baksi K (1989) Spectrophotometric determination of salicylic acid in pharmaceutical formulations using copper(II) acetate as a colour developer. Analyst 110:739–741
Sena MM, Fernandes JCB, Rover L, Poppi RJ, Kubota LT (2000) Application of two- and three-way chemometrics methods in the study of acetylsalicylic acid and ascorbic acid mixtures using ultraviolet spectrophotometry. Anal Chim Acta 409(1-2):159–170. https://doi.org/10.1016/S0003-2670(00)00707-8
Villari A, Micah N, Frest M, Puglisi G (1994) Spectrofluorimetry at zero angle: determination of salicylic acid in an acetylsalicylic acid pharmaceutical formulation. Analvst 119(7):1561–1565. https://doi.org/10.1039/an9941901561
Pena AM, Salinas F, Meras ID (1988) Simultaneous determination of salicyclic and salicyluric acids in urine by first-derivative synchronous fluorescence spectroscopy. Anal Chem 60(22):2493–2496. https://doi.org/10.1021/ac00173a012
Buskin JN, Upton RA, Williams RL (1982) Improved liquid-chromatography of aspirin, salicylate, and salicyluric acid in plasma, with a modification for determining aspirin metabolites in urine. Clin Chem 28:1200–1203
Sun LJ, Pan ZQ, Xie J, Liu XJ, Sun FT, Song FM, Bao N, Gu HY (2013) Electrocatalytic activity of salicylic acid on Au@Fe3O4 nanocomposites modified electrode and its detection in tomato leaves infected with Botrytis cinerea. J Electroanal Chem 706:127–132. https://doi.org/10.1016/j.jelechem.2013.07.038
Wang Z, Wei F, Liu SY, Xu Q, Huang JY, Dong XY, Yu JH, Yang Q, Zhao YD, Chen H (2010) Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles-modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 80(3):1277–1281. https://doi.org/10.1016/j.talanta.2009.09.023
Wang Z, Ai F, Xu Q, Yang Q, Yu JH, Huang WH, Zhao YD (2010) Electrocatalytic activity of salicylic acid on the platinum nanoparticles modified electrode by electrochemical deposition. Colloid Surface B 76(1):370–374. https://doi.org/10.1016/j.colsurfb.2009.10.038
Chrzescijanska E, Wudarska E, Kusmierek E, Rynkowski J (2014) Study of acetylsalicylic acid electroreduction behavior at platinum electrode. J Electroanal Chem 713:17–21. https://doi.org/10.1016/j.jelechem.2013.11.015
Park J, Eun C (2016) Electrochemical behavior and determination of salicylic acid at carbon-fiber electrodes. Electrochim Acta 194:346–356. https://doi.org/10.1016/j.electacta.2016.02.103
Papouchado L, Petrie G, Adams RN (1972) Anodic-oxidation pathways of phenolic compounds: Part 1. Anodic hydroxylation reactions. J Electroanal Chem 38(2):389–395. https://doi.org/10.1016/S0022-0728(72)80349-8
Koile RC, Johnson DC (1979) Electrochemical removal of phenolic films from a platinum anode. Anal Chem 51(6):741–744. https://doi.org/10.1021/ac50042a037
Lupu S, Ion I, Ion AC (2009) Voltammetric determination of phenol at platinum electrodes modified with polypyrrole doped with ferricyanide. Rev Roum Chim 54:351–357
Alizadeh T, Amjadi S (2017) Indirect voltammetric determination of nicotinic acid by using a graphite paste electrode modified with reduced graphene oxide and a molecularly imprinted polymer. Microchim Acta 184:2687–2695
Alizadeh T, Ganjali MR, Rafie F (2017) Trace level and highly selective determination of urea in various real samples based upon voltammetric analysis of diacetyl monoxime-urea reaction product on the carbon nanotube/carbon paste electrode. Anal Chim Acta 974:54–62. https://doi.org/10.1016/j.aca.2017.04.039
Raoof JB, Ojani R, Kolbadinezhad M (2009) Voltammetric sensor for glutathione determination based on ferrocene-modified carbon paste electrode. J Solid State Electrochem 13(9):1411–1416. https://doi.org/10.1007/s10008-008-0690-4
Alizadeh T, Ganjali MR, Rafiei F, Akhoundian M (2017) Synthesis of nano-sized timolol-imprinted polymer via ultrasonication assisted suspension polymerization in silicon oil and its use for the fabrication of timolol voltammetric sensor. Mater Sci Eng C 77:300–307. https://doi.org/10.1016/j.msec.2017.03.168
Morales GR, Silva TR, Galicia L (2003) Carbon paste electrodes electrochemically modified with cyclodextrin. J Solid State Electrochem 7:355–360
Alizadeh T, Jamshidi F (2015) Synthesis of nanosized sulfate-modified α-Fe2O3 and its use for the fabrication of all-solid-state carbon paste pH sensor. J Solid State Electrochem 19(4):1053–1062. https://doi.org/10.1007/s10008-014-2716-4
Tsuji E, Imanishi A, Fukui K, Nakato Y (2011) Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution. Electrochim Acta 56(5):2009–2016. https://doi.org/10.1016/j.electacta.2010.11.062
Das D, Sen PK, Das K (2008) Mechanism of potentiostatic deposition of MnO2 and electrochemical characteristics of the deposit in relation to carbohydrate oxidation. Electrochim Acta 54(2):289–295. https://doi.org/10.1016/j.electacta.2008.07.082
Sun W, Wang X, Wang W, Lu Y, Xi J, Zheng W, Wu F, Ao H, Li G (2015) Electrochemical DNA sensor for Staphylococcus aureus nuc gene sequence with zirconia and graphene modified electrode. J Solid State Electrochem 19(8):2431–2438. https://doi.org/10.1007/s10008-015-2893-9
Guerrini E, Vallini S, Colombo A, Trasatti SP, Trasatti S (2014) Anodic films containing zirconia nanoparticles for corrosion protection of AA1050 aluminum alloy. J Solid State Electrochem 18(5):1457–1468. https://doi.org/10.1007/s10008-013-2274-1
Zhao YH, Du QQ, Cao XF, Chi B, Zhang J, Zhang CM, Liu T, Wang XJ, Su YG (2012) Electronic structure and electrocatalytic activity of cerium-doped tantalum oxide. J Electroanal Chem 681:139–143. https://doi.org/10.1016/j.jelechem.2012.06.003
Doménech A, Aucejo R, Alarcón J, Navarro P (2004) Electrocatalysis of the oxidation of methylene dioxyamphetamines at electrodes modified with cerium-doped zirconias. Electrochem Commun 6(7):719–723. https://doi.org/10.1016/j.elecom.2004.05.013
Chen CH, Chiou YJ, Liou WJ, Lin WS, Lin HM, Wu SH (2011) Synthesis and electrocatalysis application of hybrid platinum/cerium oxide/multi-walled carbon nanotubes. Funct Mater Lett 4(03):295–298. https://doi.org/10.1142/S1793604711002032
Duan T, Chen Y, Wen Q, Duan Y (2015) Different mechanisms and electrocatalytic activities of Ce ion or CeO2 modified Ti/Sb–SnO2 electrodes fabricated by one-step pulse electrocodeposition. RSC Adv 5(25):19601–19612. https://doi.org/10.1039/C5RA01876E
Feng JX, Ye SH, Xu H, Tong YX, Li GR (2016) Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction. Adv Mater 28(23):4698–4703. https://doi.org/10.1002/adma.201600054
Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, Comelli G, Rosei R (2005) Electron localization determines defect formation on ceria substrates. Science 309(5735):752–755. https://doi.org/10.1126/science.1111568
Porter DL, Evans AG, Heuer AH (1979) Transformation-toughening in partially-stabilized zirconia (PSZ). Acta Met 27(10):1649–1654. https://doi.org/10.1016/0001-6160(79)90046-4
Chang JP, Lin YS, Chu K (2001) Rapid thermal chemical vapor deposition of zirconium oxide for metal-oxide-semiconductor field effect transistor application. J Vacuum Sci Tech B 19(5):1782–1787. https://doi.org/10.1116/1.1396639
Doménech A, Alarcón J (2007) Microheterogeneous electrocatalytic chiral recognition at monoclinic vanadium-doped zirconias: enantioselective detection of glucose. Anal Chem 79(17):6742–6751. https://doi.org/10.1021/ac070623w
Tekeli S, Kayış A, Gürü M (2008) Microstructural, mechanical and electrical properties of alumina-doped cubic zirconia (c-ZrO2). J Solid State Electrochem 12(7-8):791–797. https://doi.org/10.1007/s10008-008-0530-6
Doménech A, Montoya N, Alarcón J (2012) Electrochemical characterization of praseodymium centers in PrxZr1−xO2 zirconias using electrocatalysis and photoelectrocatalysis. J Solid State Electrochem 16(3):963–975. https://doi.org/10.1007/s10008-011-1470-0
Alizadeh T, Hamidia N, Ganjali MR, Nourozi P (2017) Development of a highly selective and sensitive electrochemical sensor for Bi3+determination based on nano-structured bismuth-imprinted polymer modified carbon/carbon nanotube paste electrode. Sensors Actuators B Chem 245:605–614. https://doi.org/10.1016/j.snb.2017.02.024
Alizadeh T (2012) Application of electrochemical impedance spectroscopy and conventional rebinding experiments for the investigation of recognition characteristic of bulky and nano-sized imprinted polymers. Mater Chem Phys 135(2-3):1012–1023. https://doi.org/10.1016/j.matchemphys.2012.06.007
Alizadeha T, Mirzagholipur S (2015) An outstandingly sensitive enzyme-free glucose sensor prepared by co-deposition of nano-sized cupric oxide and multi-walled carbon nanotubes on glassy carbon electrode. Biochem Eng J 97:81–91. https://doi.org/10.1016/j.bej.2015.02.011
Doménech A, Torres FJ, Alarcón J (2004) Electrochemistry of vanadium-doped ZrSiO4 site-selective electrocatalytic effect on nitrite oxidation. Electrochim Acta 49:4623–4632
Torriero AAJ, Lucoa JM, Sereno L, Rabaa J (2004) Voltammetric determination of salicylic acid in pharmaceuticals formulations of acetylsalicylic acid. Talanta 62(2):247–254. https://doi.org/10.1016/j.talanta.2003.07.005
Neumayr M, Frxednch O, Sontag G, Plttner F (1993) Flow-injection analysis with electrochemical detection for determination of salicylic acid in pharmaceutical preparations. Anal Chim Acta 273:469–475
Zhang WD, Xu B, Hong YX, Yu YX, Ye JS, Zhang JQ (2010) Electrochemical oxidation of salicylic acid at well-aligned multiwalled carbon nanotube electrode and its detection. J Solid State Electrochem 14(9):1713–1718. https://doi.org/10.1007/s10008-010-1014-z
Kubota LT, Milagres BG, Gouvea F, Neto GO (1996) A modified carbon paste electrode with silica gel coated with Meldola’s blue and salicylate hydroxylase as a biosensor for salicylate. Anal Lett 29(6):893–910. https://doi.org/10.1080/00032719608001442
Gualandi I, Scavetta E, Zappoli S, Tonelli D (2011) Electrocatalytic oxidation of salicylic acid by a cobalt hydrotalcite-like compound modified Pt electrode. Biosens Bioelectron 26(7):3200–3206. https://doi.org/10.1016/j.bios.2010.12.026
Petrek J, Havel L, Petrlova J, Adam V, Potesil D, Babula P, Kizek R (2007) Analysis of salicylic acid in willow barks and branches by an electrochemical method. Russ J Plant Physiol 54(4):553–558. https://doi.org/10.1134/S1021443707040188
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Alizadeh, T., Nayeri, S. Electrocatalytic oxidation of salicylic acid at a carbon paste electrode impregnated with cerium-doped zirconium oxide nanoparticles as a new sensing approach for salicylic acid determination. J Solid State Electrochem 22, 2039–2048 (2018). https://doi.org/10.1007/s10008-018-3907-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3907-1