Abstract
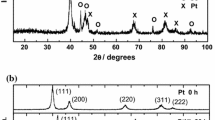
Carbon-supported Pd, Pt, Pt1Pd1, and Pt3Pd1 electrocatalysts were prepared by metal ion chemical reduction with borohydride. The electrocatalysts were analyzed by EDS, HRTEM, and XRD techniques. The EDS spectra showed that the actual compositions of the materials were close to the nominal compositions. The XRD measurements revealed that the materials were crystalline, and no oxide peaks were observed. The HRTEM micrographs showed homogeneous particle distributions on the carbon, with Gaussian particle size distributions. The mean particle sizes were taken as the maxima of the Gaussian distributions. All the materials presented electrochemical activities for the ethanol oxidation reaction, with the exception of Pd in acid media. Pt showed poor performance in alkaline media. The poisoning rate was determined by cyclic voltammetry (i forward/i backward ratios), as well as by chronoamperometry experiments, both of which indicated a low poisoning rate for Pd in alkaline medium and higher poisoning rates as the Pt content increased in the PtPd electrocatalyst








Similar content being viewed by others
References
Lamy C (2016) From hydrogen production by water electrolysis to its utilization in a PEM fuel cell or in a SO fuel cell: some considerations on the energy efficiencies. Int J Hydrogen Energy 41(34):15415–15425. https://doi.org/10.1016/j.ijhydene.2016.04.173
Lamy C, Rousseau S, Belgsir EM, Coutanceau C, Léger J-M (2004) Recent progress in the direct ethanol fuel cell: development of new platinum–tin electrocatalysts. Electrochim Acta 49(22-23):3901–3908. https://doi.org/10.1016/j.electacta.2004.01.078
Zhou W, Zhou Z, Song S, Li W, Sun G, Tsiakaras P, Xin Q (2003) Pt based anode catalysts for direct ethanol fuel cells. Appl Catal B 46(2):273–285. https://doi.org/10.1016/S0926-3373(03)00218-2
Antolini E (2007) Catalysts for direct ethanol fuel cells. J Power Sources 170(1):1–12. https://doi.org/10.1016/j.jpowsour.2007.04.009
Ong BC, Kamarudin SK, Basri S (2017) Direct liquid fuel cells: a review. Int J Hydrogen Energy 42(15):10142–11015. https://doi.org/10.1016/j.ijhydene.2017.01.117
Carrera-Cerritos R, Fuentes-Ramírez R, Cuevas-Muñiz FM, Ledesma-García J, Arriaga LG (2014) Performance and stability of Pd nanostructures in an alkaline direct ethanol fuel cell. J Power Sources 269:370–378. https://doi.org/10.1016/j.jpowsour.2014.06.161
Erikson H, Liik M, Sarapuu A, Marandi M, Sammelseg V, Tammeveski K (2013) Electrocatalysis of oxygen reduction on electrodeposited Pd coatings on gold. J Electroanal Chem 691:35–41. https://doi.org/10.1016/j.jelechem.2012.12.018
Modibedi RM, Mehlo TM, Ozoemena KI, Mathe MK (2015) Preparation, characterization and application of Pd/C nanocatalyst in passive alkaline direct ethanol fuel cells (ADEFC). Int J Hydrog Energy 40(45):15605–15612. https://doi.org/10.1016/j.ijhydene.2015.08.113
Qian K, Hao F, Wei S, Wang Y, Ge C, Chen P, Zhang Y (2017) Synthesis of well-dispersed Pt-Pd nanoparticles stabilized by silsesquioxanes with enhanced catalytic activity for formic acid electrooxidation. Solid State Electrochem 21(1):297–304. https://doi.org/10.1007/s10008-016-3334-0
Luo W, Zhou H, Fu C, Huang Z, Gao N, Kuang Y (2016) Preparation and characterization of porous sponge-like Pd@Pt nanotubes with high catalytic activity for ethanol oxidation. Mater Lett 173:43–46. https://doi.org/10.1016/j.matlet.2016.03.012
Rana M, Patil PK, Chhetri M, Dileep K, Datta R, Gautam UK (2016) Pd–Pt alloys nanowires as support-less electrocatalyst with high synergistic enhancement in efficiency for methanol oxidation in acidic medium. J Colloid Interface Sci 463:99–106. https://doi.org/10.1016/j.jcis.2015.10.042
Antolini E (2009) Palladium in fuel cell catalysis. Energy Environ Sci 2(9):915–931. https://doi.org/10.1039/b820837a
Kadirgan F, Beyhan S, Atilan T (2009) Preparation and characterization of nano-sized Pt–Pd/C catalysts and comparison of their electro-activity toward methanol and ethanol oxidation. Int J Hydrogen Energy 34(10):4312–4320. https://doi.org/10.1016/j.ijhydene.2009.03.024
Wang Y, Zou S, Cai W-B (2015) Recent advances on electro-oxidation of ethanol on Pt- and Pd-based catalysts: from reaction mechanisms to catalystic materials. Catalysts 5(3):1507–1534. https://doi.org/10.3390/catal5031507
Mao H, Huang T, Yu A (2016) Surface noble metal modified PdM/C (M = Ru, Pt, Au) as anode catalysts for direct ethanol fuel cells. J Alloys Compounds 676:390–396. https://doi.org/10.1016/j.jallcom.2016.03.200
Monyoncho EA, Ntais S, Soares F, Woo TK, Baranova EA (2015) Synergetic effect of palladium-ruthenium nanostructures for ethanol electrooxidation in alkaline media. J Power Sources 287:139–149. https://doi.org/10.1016/j.jpowsour.2015.03.186
Vecchio CL, Sebastián D, Alegre C, Aricò AS, Baglio V (2017) Carbon-supported Pd and Pd-Co cathode catalysts for direct methanol fuel cells (DMFCs) operating with high methanol concentration. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2017.02.042
Mandal K, Bhattacharjee D, Roy PS, Bhattacharya SK, Dasgupta S (2015) Room temperature synthesis of Pd–Cu nanoalloy catalyst with enhanced electrocatalytic activity for the methanol oxidation reaction. Appl Catal A Gen 492:100–106. https://doi.org/10.1016/j.apcata.2014.12.012
Xu C, Tian Z, Shen P, Jiang SP (2008) Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochim Acta 53(5):2610–2618. https://doi.org/10.1016/j.electacta.2007.10.036
Yang Z, Wang X, Kang X, Zhang S, Guo Y (2017) The PtPdAg/C electrocatalyst with Pt-rich surfaces via electrochemical dealloying of Ag and Pd for ethanol oxidation. Electrochim Acta 236:72–81. https://doi.org/10.1016/j.electacta.2017.03.165
Shang C, Hong W, Wang J, Wang W (2015) Carbon supported trimetallic nickel-palladium-gold hollow nanoparticles with superior catalytic activity for methanol electrooxidation. J Power Sources 285:12–15. https://doi.org/10.1016/j.jpowsour.2015.03.092
Zhu F, Ma G, Bai Z, Hang R, Tang B, Zhang Z, Wang X (2017) High activity of carbon nanotubes supported binary and ternary Pd-based catalysts for methanol, ethanol and formic acid electro-oxidation. J Power Sources 242:610–620
Zhu F, Wang M, He Y, Ma G, Zhang Z, Wang X (2014) A comparative study of elemental additives (Ni, Co and Ag) on electrocatalytic activity improvement of PdSn-based catalysts for ethanol and formic acid electro-oxidation. Electrochim Acta 148:291–301. https://doi.org/10.1016/j.electacta.2014.10.062
Wang M, Ma Z, Li R, Tang B, Bao X-Q, Zhang Z, Wang X (2017) Novel flower-like PdAu(Cu) anchoring on a 3D rGO-CNT sandwich-stacked framework for highly efficient methanol and ethanol electro-oxidation. Electrochim Acta 227:330–344. https://doi.org/10.1016/j.electacta.2017.01.046
Carvalho LL, Colmati F, Tanaka AA (2017) Nickel-palladium electrocatalysts for methanol, ethanol, and glycerol oxidation reactions. Int J Hydrogen Energy 42(25):16118–16126. https://doi.org/10.1016/j.ijhydene.2017.05.124
Machida K, Enyo M (1987) In situ X-ray-diffraction study of hydrogen entry into Pd and Pd–Au alloy electrodes during anodic HCHO oxidation. J Electrochem Soc 134(6):1472–1474. https://doi.org/10.1149/1.2100693
Zhou WJ, Song SQ, Li WZ, Zhou ZH, Sun GQ, Xin Q, Douvartzides S, Tsiakaras P (2005) Direct ethanol fuel cells based on PtSn anodes: the effect of Sn content on the fuel cell performance. J Power Sources 140(1):50–58. https://doi.org/10.1016/j.jpowsour.2004.08.003
Montiel MA, Vidal-Iglesias FJ, Montiel V, Solla-Gulón J (2017) Electrocatalysis on shape-controlled metal nanoparticles: progress in surface cleaning methodologies. Curr Opin Electrochem 1(1):34–39. https://doi.org/10.1016/j.coelec.2016.12.007
Peng L, Duyne RPV, Marks LD (2016) Compositional inhomogeneity and corner enrichment of Pt in Pt/Pd bimetallic nanoparticles. J Phys Chem C 37:21069–21075
Cao S, Tao FF, Li Y, Yu J (2016) Size and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem Soc Rev 45(17):4747–4765. https://doi.org/10.1039/C6CS00094K
Yang G, Zhou Y, Pan H-B, Zhu C, Fu S, Wai CM, Du D, Zhu J-J, Lin Y (2016) Ultrasonic-assisted synthesis of Pd–Pt/carbon nanotubes nanocomposites for enhanced electro-oxidation of ethanol and methanol in alkaline medium. Ultrasonics Chem 28:192–198
Lee D-W, Jin M-J, Lee Y-J, Park J-H, Lee C-B, Park J-S (2016) Reducing-agent-free instant synthesis of carbon-supported Pd catalysts in a green leidenfrost droplet reactor and catalytic activity in formic acid dehydrogenation. Sci Rep 6(1):26474–26483. https://doi.org/10.1038/srep26474
Baylet A, Marécot P, Duprez D, Castellazzi P, Groppi G, Forzatti P (2011) In situ raman and in situ XRD analysis of PdO reduction and Pd oxidation supported on γ-Al2O3 catalyst under different atmosphere. Phys Chem Chem Phys 13(10):4607–4313. https://doi.org/10.1039/c0cp01331e
Sekiguchi Y, Hayashi Y, Takizawa H (2011) Synthesis of palladium nanoparticles and palladium/spherical carbon composite particles in the solid-liquid system of palladium oxide-alcohol by microwave irradiation. Mater Trans 5:1048–1052
Lin S-C, Chen J-Y, Hsieh Y-F, Wu P-W (2011) A facile route to prepare PdPt alloys for ethanol electro-oxidation in alkaline electrolyte. Mater Lett 65(2):215–218. https://doi.org/10.1016/j.matlet.2010.10.006
Wang J, Cheng N, Banis MN, Xiao B, Riese A, Sun X (2015) Comparative study to understand the intrinsic properties of Pt and Pd catalysts for methanol and ethanol oxidation in alkaline media. Electrochim Acta 185:267–275. https://doi.org/10.1016/j.electacta.2015.10.151
Oezaslan M, Herrmann A-K, Werheid M, Frenkel AI, Nachtegaal M, Dosche C, Bonnaud L, Yilmaz HC, Kühn L, Rhiel E, Gaponik N, Eychmüller A, Schmidt TJ (2017) Structural analysis and electrochemical properties of bimetallic palladium–platinum aerogels prepared by a two-step gelation process. Chem Cat Chem 9:1–12
Perales-Rondón JV, Herrero E, Solla-Gullón J, Sánchez-Sáncheza CM, Vivier V (2017) Oxygen crossover effect on palladium and platinum based electrocatalysts during formic acid oxidation studied by scanning electrochemical microscopy. J Electroanal Chem 793:218–225. https://doi.org/10.1016/j.jelechem.2016.12.049
Lee Y-W, Lee J-Y, Kwak D-H, Hwang E-T, Sohn JI, Park K-W (2015) Pd@Pt core–shell nanostructures for improved electrocatalytic activity in methanol oxidation reaction. Appl Catal B Environ 179:178–184. https://doi.org/10.1016/j.apcatb.2015.05.029
Wang X, Zhu F, He Y, Wang M, Zhang Z, Ma Z, Li R (2016) Highly active carbon supported ternary PdSnPtx (x = 0.1–0.7) catalysts for ethanol electro-oxidation in alkaline and acid media. J Colloid Interface Sci 468:200–210. https://doi.org/10.1016/j.jcis.2016.01.068
Ma L, Chu D, Chen R (2012) Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Inter J Hydrogen Energy 37(15):11185–11194. https://doi.org/10.1016/j.ijhydene.2012.04.132
Wang X, Tang B, Huang X, Ma Y, Zhang Z (2013) High activity of novel nanoporous Pd-Au catalysts for methanol electro-oxidation in alkaline media. J Alloys Com 565:120–126. https://doi.org/10.1016/j.jallcom.2013.02.170
Starz KA, Auer E, Lehmann T, Zuber R (1999) Characteristics of platinum-based electrocatalysts for mobile PEMFC applications. J Power Sources 84(2):167–172. https://doi.org/10.1016/S0378-7753(99)00333-X
Reis RGCS, Colmati F (2016) Electrochemical alcohol oxidation: a comparative study of the behavior of methanol, ethanol, propanol, and butanol on carbon-supported PtSn, PtCu, and Pt nanoparticles. Solid State Electrochem 20(9):2559–2567. https://doi.org/10.1007/s10008-016-3323-3
Colmati F, Paganin VA, Gonzalez ER (2006) Effect of operational parameters of mini-direct methanol fuel cells operating at ambient temperature. J Appl Electrochem 36(1):17–23. https://doi.org/10.1007/s10800-005-9019-5
Yi Q, Niu F, Sun L (2011) Fabrication of novel porous Pd particles and their electroactivity towards ethanol oxidation in alkaline media. Fuel 90(8):2617–2623. https://doi.org/10.1016/j.fuel.2011.03.038
Kokoh KB, Hahn F, Belgsir EM, Lamy C, Andrade AR, Olivi P, Motheo AJ, Tremiliosi-Filho G (2004) Electrocatalytic oxidation of acetaldehyde on Pt alloy electrodes. Electrochim Acta 49(13):2077–2083. https://doi.org/10.1016/j.electacta.2003.11.015
Lopes T, Antolini E, Gonzalez ER (2008) Carbon supported Pt-Pd alloy as an ethanol tolerant oxygen reduction electrocatalysts for direct ethanol fuel cells. Int J Hydrogen Energy 33(20):5563–5570. https://doi.org/10.1016/j.ijhydene.2008.05.030
Rocha TA, Linares JJ, Colmati F, Ciapina EG, Congalez ER (2012) Electrocatalytic activity of platinum-niobium nanoparticles for ethanol oxidation. J Electrochem Soc 159(10):F650–F658. https://doi.org/10.1149/2.040210jes
Jiang J, Kucernak A (2003) Electrooxidation of small organic molecules on mesoporous precious metal catalysts II: CO and methanol on platinum/ruthenium alloy. J Electroanal Chem 543(2):187–199. https://doi.org/10.1016/S0022-0728(03)00046-9
Guo JW, Zhao TS, Prabhuram J, Chen R, Wong CW (2005) Preparation and characterization of a PtRu/C nanocatalyst for direct methanol fuel cells. Electrochim Acta 51(4):754–763. https://doi.org/10.1016/j.electacta.2005.05.056
Chen C-C, Lin C-L, Chen L-C (2015) Functionalized carbon nanomaterial supported palladium nano-catalysts for electrocatalytic glucose oxidation reaction. Electrochim Acta 152:408–416. https://doi.org/10.1016/j.electacta.2014.11.116
Chen C-C, Lin C-L, Chen L-C (2015) A binary palladium-bismuth nanocatalyst with high activity and stability for alkaline glucose electrooxidation. J Power Sources 287:323–333. https://doi.org/10.1016/j.jpowsour.2015.04.083
Ning L, He X, Zhu A, Deng M, Zhang Q, Liu Q (2017) Novel H-PdSnNi catalyst with enhanced ethanol electrooxidation performance in alkaline medium. Electrochim Acta. https://doi.org/10.1016/j.electacta.2017.10.112
Tripković AV, Popović KDJ, Lović JD (2001) The influence of the oxygen-containing species on the electrooxidation of the C1–C4 alcohols at some platinum single crystal surfaces in alkaline solution. Electrochim Acta 46(20-21):3163–3173. https://doi.org/10.1016/S0013-4686(01)00608-9
Acknowledgements
We would like to thank Tatiane Oliveira dos Santos, from the Microscopy Laboratory (LABMIC) of the Federal University of Goiás, Goiânia, Brazil, for assisting with use of the JEOL JEM-2010 HRTEM microscope.
Funding
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grants # 554569/2010-8 and 309066/2013-1, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
• Comparison of the electrochemical activities of Pd, Pt, and PdPt electrocatalysts towards the ethanol oxidation reaction in acid and alkaline media.
• Calculation of Pd and Pt-Pd poisoning rates using cyclic voltammetry and chronoamperometry experiments.
Rights and permissions
About this article
Cite this article
Carvalho, L.L., Tanaka, A.A. & Colmati, F. Palladium-platinum electrocatalysts for the ethanol oxidation reaction: comparison of electrochemical activities in acid and alkaline media. J Solid State Electrochem 22, 1471–1481 (2018). https://doi.org/10.1007/s10008-017-3856-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3856-0