Abstract
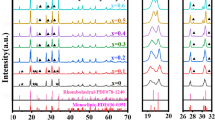
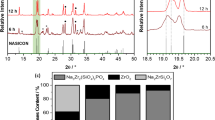
Na2Ti3O7 ceramic materials have been widely used in sodium-ion battery applications with relative good results; however, there are still several studies that might be carried out in the improvement of the Na2Ti3O7 properties and the overall batteries’ performance. In this direction, we used sonochemical method following a thermal treatment in order to synthetized pure phase Na2Ti3O7 nanopowders. X-ray diffraction characterization revealed that Na2Ti3O7 is the primary phase in nanopowders and ceramic sample; although, a high level of amorphization was observed in the sonicated nanopowder without heat treatment process. Nanopowder-prepared ceramic sample showed a crystallite size of 50 nm after sintering at 900 °C for 1 h. The specific surface area, pore volume, and pore size were obtained from the B.E.T. measurements, being 51 m2 g−1, 0.07 cm3 g−1, and 55 Å, respectively. The capacitance values of the nanopowder-prepared ceramic sample were in the order of microfarad. The total energy of the system was used to determine relaxation time of the sample (τ 0 = 31 ms).




Similar content being viewed by others
References
Kim KT, Ali G, Chung KY, Yoon CS, Yashiro H, Sun YK, Lu J, Amine K, Myung ST (2014) Anatase titania nanorods as an intercalation anode material for rechargeable sodium batteries. Nano Lett 14:416–422
Shirpour M, Cabana J, Doeff M (2013) New materials based on a layered sodium titanate for dual electrochemical Na and Li intercalation systems. Energy Environ Sci 6:2538–2547
Sun Y, Zhao L, Pan H, Lu X, Gu L, Hu YS, Li H, Armand M, Ikuhara Y, Chen LQ (2013) Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries. Nat Commun 4:1870
Hayashi A, Noi K, Sakuda A, Tatsumisago M (2012) Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat Commun 3:856
Wenzel S, Hara T, Janek J, Adelhelm P (2011) Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Environ Sci 4:3342–3345
Zhang B, Yu Y, Liu Y, Huang ZD, He YB, Kim JK (2013) Percolation threshold of graphene nanosheets as conductive additive in Li4Ti5O12 anode of Li-ion batteries. Nanoscale 5:2100–2106
Senguttuvan P, Rousse G, Seznec V, Tarascon JM, Palacin MR (2011) Na2Ti3O7: lowest voltage ever reported oxide insertion electrode for sodium ion batteries. Chem Mater 23:4109–4111
Zarrabeitia M, Castillo-Martínez E, Lopez Del Amo JM, Eguía-Barrio A, Muñoz-Márquez MA, Rojo T, Casas-Cabanas M (2016) Towards environmentally friendly Na-ion batteries: Moisture and water stability of Na2Ti3O7. J Power Sources 324:378–387
Kobayashi T, Yamada A, Kanno R (2008) Interfacial reactions at electrode/electrolyte boundary in all solid-state lithium battery using inorganic solid electrolyte, thio-LISICON. Electrochim Acta 53:5045–5050
Zhiming Z, Hongmei X, Fan Z, Xiaolong Z, Yongbing T (2016) Solvothermal synthesis of Na2Ti3O7 nanowires embedded in 3D graphene networks as an anode for high-performance sodium-ion batteries. Electrochim Acta 211:430–436
Li M, Xiao X, Fan X, Huang X, Liu Y, Chen L (2017) Carbon coated sodium-titanate nanotube as an advanced intercalation anode material for sodium-ion batteries. J Alloys Compd 712:365–372
Ghosh S, Kee Y, Okada S, Barpanda P (2015) Energy-savvy solid-state and sonochemical synthesis of lithium sodium titanate as an anode active material for Li-ion batteries. J Power Sources 296:276–281
Leyet Y, Guerrero F, Anglada-Rivera J, Wilson D, Peña-Garcia R, Delgado A, Guerra Y, Padrón-Hernández E, Pérez de la Cruz J (2017) Anomalous ferromagnetic response in Na2Ti3O7 nanopowder obtained by the sonochemical method. Mater Res Express 4:045010
Rudola A, Sharma N, Balaya P (2015) Introducing a 0.2 V sodium-ion battery anode: The Na2Ti3O7 to Na3-xTi3O7 pathway. Electrochem Commun 61:10–13
Leyet Y, Guerrero F, Pérez-Delfín E, Pérez de la Cruz J, Eiras JA (2012) Characterization of nanostructured ceramics prepared by both high energy ball milling and fast firing sintering processes. Phase Transit 85:136–148
Shen K, Wagemaker M (2014) Na2+xTi6O13 as potential negative electrode material for Na-ion batteries. Inorg Chem 53:8250–8256
Sauvet AL, Baliteau S, Lopez C, Fabry P (2004) Synthesis and characterization of sodium titanates Na2Ti3O7 and Na2Ti6O13. J Solid State Chem 177:4508–4515
Dynarowska M, Kotwiński J, Leszczynska M, Marzantowicz M, Krok F (2017) Ionic conductivity and structural properties of Na2Ti3O7 anode material. Solid State Ionics 301:35–42
Buchelli W, Jimenez R, Sanz J, Várez A (2012) The log(σ) vs. log(ω) derivate plot used to analyze the ac conductivity. Application to fast Li+ ion conductors with perovskite structure. Solid State Ionics 227:113–118
Ganesh V, Pitchumani S, Lakshminarayanan V (2006) New symmetric and asymmetric supercapacitors based on high surface area porous nickel and activated carbon. J Power Sources 158:1523–1532
Acknowledgments
This work has been supported by the Brazilian Agencies CNPq, FINEP, FACEPE, FAPEAM, MCTI and CAPES (99999.008454/2014-00).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leyet, Y., Guerrero, F., Anglada-Rivera, J. et al. Synthesis of Na2Ti3O7 nanoparticles by sonochemical method for solid state electrolyte applications. J Solid State Electrochem 22, 1315–1319 (2018). https://doi.org/10.1007/s10008-017-3697-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3697-x