Abstract
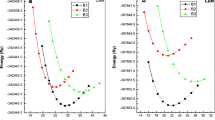
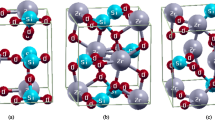
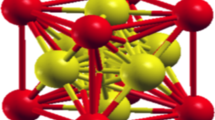
The present work aims at the study of structural, elastic, electronic, and thermodynamic properties of transition metal nitrides: ReN and MoN in the zinc-blende (B3) phase. The plane wave pseudopotential and norm-conserving pseudopotential have been applied in Quantum-Espresso code based on density-functional theory (DFT). The results show a first-order phase transition from B3 to B1 (rock-salt) structure at 42 GPa and 2.5 GPa for ReN and MoN respectively. The elastic behaviors of these compounds are also unfolded in this work. The brittleness of the ReN and ductility of MoN is identified with the help of Pugh’s index and Poisson’s ratio. The strong anisotropic behaviors of both compounds are detected under the influence of pressure. The electronic and bonding features of proposed compounds are evaluated by means of band structures, the density of states (DOS), Fermi surface, and charge density plots. The obtained results forecast the metallic behavior and ionic bonding of ReN and MoN in both phases: B3 and B1. Additionally, various thermodynamic properties are also investigated under high pressures and temperatures (from 0 to 2000 K). Conceivably, these properties are reported for the first time in the B3 structure of these compounds and will be useful for many applications in modern technologies as well.

















Similar content being viewed by others
Change history
19 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00894-020-04664-2
References
Wu ZG, Chen XJ, Struzhkin VV, Cohen RE (2005). Phys Rev B 71:214103
Friedrich A, Winkler B, Bayarjargal L, Morgenroth W, Juarez Arellano EA, Milman V, Refson K, Kunz M, Chen K (2010). Phys Rev Lett 105:085504
Yamanaka S, Hotehama K, Kawaji H (1998). Nature (London) 392:580
Zerr A, Miehe G, Boehler R (2003). Nat Mater 2:185
Soignard E, Shebanova O, McMillan PF (2007). Phys Rev B 75:014104
Dar SA, Srivastava V, Sakalle UK (2017). J Electron Mater. https://doi.org/10.1007/s11664-017-5731-2
Ali Z, Ahmad I, Amin B (2012). Intermetallics 13:287
Lee JH, Rabe KM (2010). Phys Rev Lett 104:207204
Srinvasan G, Rasmussen ET, Jevin BJ, Hayes R (2002). Phys Rev B 65:134402
Van Doorn RHE, Bouwmeester HJM, Burgraap AJ (1998). Solid State Ionics 111:263
Derras M, Hamad N, Derras M, Gessoum A (2013). Results Phys 3:219
Ali Z, Ahmad I, Khan I, Amin B (2012). Intermetallics 31:287
Ali Z, Khan I, Ahmad I, Naeem S, Rahnamaye Aliabad HA, Asadabidi SJ, Zhang Z (2013). Phys B 423:16
Ali Z, Shafiq M, Asadabidi SJ, Rahnamaya HA, Abid A, Khan I, Ahmad I (2014). Comput Mater Sci 81:141
Wang Y, Yao T, Li H, Lian J, Li J, Li Z, Zhang J, Gou H (2012). Comput Mater Sci 56:116–121
Patil SKR, Mangale NS, Khare SV, Marsillac S (2008). Thin Solid Films 517:824–827
Kaner RB, Gilman JJ, Tolbert SH (2005). Science 308:1268–1269
Benyelloul K, Seddik L, Bouhadda Y, Bououdina M, Aouragb H, Khodja K (2017). J Phys Chem Solids 111:229–237
Lei HR, Zhu J, Hao YJ, Zhang L, Yu BR, Chen LQ, Zou YC (2015). Physica B 458:124–131
Rajeswarapalanichamy R, Kavitha M, Sudha Priyanga G, Iyakutti K (2015). J Phys Chem Solids 78:118–126
Asvini meenaatci AT, Rajeswarapalanichamy R, Iyakutti K (2011). Physica B 406:3303–3307
Li YL, Zeng Z (2009). Solid State Commun 149:1591–1595
Wang HY, Yan P, Xu L, Zhou DW (2019). Phase Transit. https://doi.org/10.1080/01411594.2019.1610759
Bannikov VV, Shein IR, Ivanovskii AL (2011). Phys Status Solidi B 248:1369–1374
Zheng X, Wang H, Yu X, Feng J, Shen X, Zhang S, Yang R, Zhou X, Xu Y, Yu R, Xiang H, Hu Z, Jin C, Zhang R, Wei S, Han J, Zhao Y, Li H, Wang S (2018). Appl Phys Lett 113:221901
Chen W, Jiang JZ (2010). J Alloys Compd 499:243–254
Runge E, Gross EKU (1984). Phys Rev Lett 52:997
Giannozzi P, Baroni S, Banoni N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I (2009). J Phys Condens Matter 21:395502
Troullier N, Martins JL (1991). Phys Rev B 43:1993
Perdew JP, Burke K, Ernzerhof M (1996). Phys Rev Lett 77:3865
Monkhorst HJ, Pack JD (1976). Phys Rev B 13:5188
Golesorkhtabar R, Pavone P, Spitaler J, Puschnig P, Draxl C (2013). Comput Phys Commun 184:1861
Suna Z, Li S, Ahujab R, Schneida JM (2004). Solid State Commun 129:589
Voigt W (1889). Ann Phys 274:573–587
Reuss A (1929). Z Ang Math Mech 9:49–58
Hill R (1953). R: Proc Phy Soc London 65:909
Haines J, Leger JM, Bocquillon G (2001). Annu Rev Mater Res 31:1
Birch F (1947). Phys Rev 71(11):809
Pugh SF (1954). Philos Mag 45:823–843
Blanco MA, Francisco E, Luaña V (2004). Comput Phys Commun 158:57
Petit AT, Dulong PL (1891). Ann Chim Phys 10:395
Acknowledgments
Author 2 is grateful to Mr. Talal Ali Al Naqbi, Line manager, Ministry of Education, UAE for his kind support and motivation.
claims and standards.
Availability of data and material
All the data, material and codes used in this work are strictly following their terms and conditions for publication
Code availability
All the codes used in the work is as per the published
Author information
Authors and Affiliations
Contributions
All authors are contributed collectively for the completion of the work.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors declare that they have no conflict of interest.
Ethics approval
This work follows all the ethics for publication.
Consent to participate
The work is carried out with the consent from responsible authorities.
Consent for publication
No objections are there from any author or affiliated institution.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The presentation of Fig. 8 was incorrect.
Highlights
• Phase transition from B3→B1 for ReN and MoN are observed with the assist of density functional theory.
• Our results show brittle/ductile nature of ReN and MoN compounds.
• Pressure dependence of elastic properties are reported for the first time.
• Electronic property calculations, predict the metallic behaviour and ionic bonding of both compounds.
• Thermodynamic properties are calculated for the first time.
Rights and permissions
About this article
Cite this article
Sarwan, M., M., F. & Singh, S. An analysis of structural phase transition and allied properties of cubic ReN and MoN compounds. J Mol Model 27, 1 (2021). https://doi.org/10.1007/s00894-020-04615-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04615-x