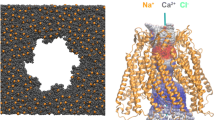
Abstract
Following our previous work, where we described the interaction of calcium with the Cx26 hemichannel, we further explore the same system by atomistic molecular dynamics simulations considering a different di-cation, magnesium. Specifically, the interaction of magnesium di-cation with the previously reported calcium binding sites (ASP2, ASP117, ASP159, GLU114, GLU119, GLU120, and VAL226) was investigated to identify similarities and differences between them. In order to do so, four extensive simulations were carried out. Two of them considered a Cx26 hemichannel embedded on a POPC bilayer with one of the di-cations and a sodium-chlorine solution. For the remaining two, no di-cations were included and a sodium-chlorine or potassium-chlorine solution was considered. Potassium has a similar atomic mass to calcium, and sodium to magnesium, but they both differ in charge (1e and 2e respectively). Magnesium and calcium, even having the same charge, showed different affinity for the explored protein. From the calcium binding sites referred above, we found that the magnesium di-cations only binds strongly to the GLU114 site of one connexin. For the sodium and potassium simulations, no specific interactions with the protein were found. Altogether, these results suggest that mass and steric effects play an important role in determining cation binding to Cx26 hemichannels.







Similar content being viewed by others
References
Trosko JE, Ruch RJ (1998) Cell-cell communication in carcinogenesis. Front Biosci 3:d208–d236. https://doi.org/10.2741/A275
Vinken MB, Vanhaecke T, Papeleu P et al (2006) Connexins and their channels in cell growth and cell death. Cell Signal 18:592–600. https://doi.org/10.1016/j.cellsig.2005.08.012
Vinken M (2015) Introduction: connexins, pannexins and their channels as gatekeepers of organ physiology. Cell Mol Life Sci 72:2775–2778. https://doi.org/10.1007/s00018-015-1958-3
Evans WH, Martin PEM (2002) Gap junctions: structure and function (Review). Mol Membr Biol 19:121–136. https://doi.org/10.1080/09687680210139839
Peracchia C (2004) Chemical gating of gap junction channels: roles of calcium, pH and calmodulin. Biochim Biophys Acta Biomembr 1662:61–80. https://doi.org/10.1016/j.bbamem.2003.10.020
Pantano S, Zonta F, Mammano F (2008) A fully atomistic model of the Cx32 connexon. PLoS One 3:1–11. https://doi.org/10.1371/journal.pone.0002614
Yeager M, Harris AL (2007) Gap junction channel structure in the early 21st century: facts and fantasies. Curr Opin Cell Biol 19:521–528. https://doi.org/10.1016/j.ceb.2007.09.001
Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC (2003) New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617
Hung A, Yarovsky I (2011) Gap junction hemichannel interactions with zwitterionic lipid, anionic lipid, and cholesterol: molecular simulation studies. Biochemistry 50:1492–1504. https://doi.org/10.1021/bi1004156
Lopez W, Ramachandran J, Alsamarah A et al (2016) Mechanism of gating by calcium in connexin hemichannels. Proc Natl Acad Sci 201609378. https://doi.org/10.1073/pnas.1609378113
Zonta F, Polles G, Zanotti G, Mammano F (2012) Permeation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J Biomol Struct Dyn 29:985–998. https://doi.org/10.1080/073911012010525027
Zhang Y, Tang W, Ahmad S et al (2005) Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci U S A 102:15201–15206. https://doi.org/10.1073/pnas.0501859102
Ebihara L, Steiner E (1993) Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol 102:59–74
Albano JMRJMR, Mussini N, Toriano R et al (2018) Calcium interactions with Cx26 hemmichannel: Spatial association between MD simulations biding sites and variant pathogenicity. Comput Biol Chem 77:331–342. https://doi.org/10.1016/j.compbiolchem.2018.11.004
Bennett BC, Purdy MD, Baker KA et al (2016) An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat Commun 7:8770. https://doi.org/10.1038/ncomms9770
Beyer EC, Berthoud VM (2017) Gap junction structure: unraveled, but not fully revealed. F1000Research 6:568. https://doi.org/10.12688/f1000research.10490.1
Ebihara L, Liu X, Pal JD (2003) Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys J 84:277–286. https://doi.org/10.1016/S0006-3495(03)74848-6
Cernak I, Radosevic P, Malicevic Z, Savic J (1995) Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnes Res 8:249–259
Heath DL, Vink R (1996) Traumatic brain axonal injury produces sustained decline in intracellular free magnesium concentration. Brain Res 738:150–153
Murphy E, Steenbergen C, Levy LA et al (1989) Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem 264:5622–5627
Helpern JA, Vande Linde AM, Welch KM et al (1993) Acute elevation and recovery of intracellular [Mg2+] following human focal cerebral ischemia. Neurology 43:1577–1581
Wood I, Albano JMR, Filho PLO et al (2018) A sumatriptan coarse-grained model to explore different environments: interplay with experimental techniques. Eur Biophys J 47:561–571. https://doi.org/10.1007/s00249-018-1278-2
Calì T, Frizzarin M, Luoni L et al (2017) The ataxia related G1107D mutation of the plasma membrane Ca2 + ATPase isoform 3 affects its interplay with calmodulin and the autoinhibition process. Biochim Biophys Acta Mol basis Dis 1863:165–173. https://doi.org/10.1016/j.bbadis.2016.09.007
Zonta F, Buratto D, Crispino G et al (2018) Cues to opening mechanisms from in silico electric field excitation of Cx26 hemichannel and in vitro mutagenesis studies in HeLa Transfectans. Front Mol Neurosci 11. https://doi.org/10.3389/fnmol.2018.00170
Abraham MJ, Murtola T, Schulz R et al (2015) Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Best RB, Zhu X, Shim J et al (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ 1 and χ 2 dihedral angles. J Chem Theory Comput 8:3257–3273. https://doi.org/10.1021/ct300400x
Albano JMR, de Paula E, Pickholz M (2018) Molecular dynamics simulations to study drug delivery systems. Molecular Dynamics. InTech. https://doi.org/10.5772/intechopen.75748
Wu Y, Tepper HL, Voth GA (2006) Flexible simple point-charge water model with improved liquid-state properties. J Chem Phys 124:024503. https://doi.org/10.1063/1.2136877
Mahoney MW, Jorgensen WL (2000) A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J Chem Phys 112:8910. https://doi.org/10.1063/1.481505
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511. https://doi.org/10.1063/1.447334
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31:1695–1697. https://doi.org/10.1103/PhysRevA.31.1695
Parrinello M, Rahman A (1982) Strain fluctuations and elastic constants. J Chem Phys 76:2662–2666. https://doi.org/10.1063/1.443248
Ewald PP (1921) Die berechnung Optischer und Elektrostatisher Gitterpotentiale. Ann Phys 64:253–287
Herce HD, Garcia AE, Darden T (2007) The electrostatic surface term:(I) periodic systems. J Chem Phys 126:124106
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load balanced, and scalable molecular simulations. J Chem Theory Comput 4:435–447
Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA (2004) PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res 32:W665–W667. https://doi.org/10.1093/nar/gkh381
Jo S, Kim T, Iyer V, Im W (2008) CHARMM GUI: a web based graphical user interface for CHARMM. J Comput Chem 29:1859–1865. https://doi.org/10.1002/jcc.20945
Wu EL, Cheng X, Jo S et al (2014) CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J Comput Chem 35:1997–2004. https://doi.org/10.1002/jcc.23702
Albano JMR (2019) Structure: Cx26 hemichannel embedded in a POPC bilayer. https://doi.org/10.5281/ZENODO.2717742
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Jones DE, Lund AM, Ghandehari H, Facelli JC (2016) Molecular dynamics simulations in drug delivery research: calcium chelation of G3.5 PAMAM dendrimers. Cogent Chem 2:1229830. https://doi.org/10.1080/23312009.2016.1229830
Funding
The Center for High Performance Computing at The Utah University provided computer resources for High Performance Computing, which has been partially funded by the NIH Shared Instrumentation Grant 1S10OD021644-01A1. JCF has been partially supported by the University of Utah Center for Clinical and Translational Science under NCATS Grant U01TR002538. MBF has been partially supported by the University of Buenos Aires Grant 20020170100456BA and PIP CONICET 11220130100377. JMRA has been partially supported by the Florencio Fiorini Foundation. MP has been partially supported by grants ANPCyT PICT2014- 3653, PIP CONICET0131-2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
ESM 1
(PDF 60.6 kb)
Rights and permissions
About this article
Cite this article
Albano, J.M.R., Facelli, J.C., Ferraro, M.B. et al. Magnesium interactions with a CX26 connexon in lipid bilayers. J Mol Model 25, 232 (2019). https://doi.org/10.1007/s00894-019-4121-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4121-5