Abstract
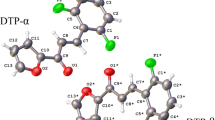
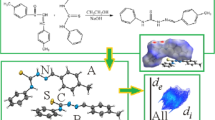
Chalcones have been reported to present biological activities that are potentialized when a sulfonamide group is attached. A comprehensive structural study was performed for arylsulfonamide chalcone N-(2-(3–4-methoxyphenyl-propanoyl)-phenyl)-benzene-sulfonamide in order to describe its supramolecular arrangement and its physicochemical properties. The molecular packing arrangement was described by X-ray diffraction and Hirshfeld surfaces (HS). Theoretical calculations were performed using density functional theory (DFT), molecular electrostatic potential (MEP) mapping, ab initio Car-Parrinelo molecular dynamics (CPMD) and the quantum theory of atoms in molecules (QTAIM). The solid-state arrangement is stabilized by C– H⋯O and C–H⋯π interactions observed on HS and MEP map. The topological analysis was evaluated by QTAIM.












Similar content being viewed by others
References
Aitipamula S, Vangala VR (2006) X-Ray crystallography and its role in understanding the physicochemical properties of pharmaceutical cocrystals. J Indian Inst Sci 97. https://doi.org/10.1007/s41745-017-0026-4
Atwood JL, Gokel GW, Barbour LJ (eds) (2017) Comprehensive supramolecular chemistry, 2nd edn. Elsevier, Amsterdam
Cacialli F, Samorì P, Silva C (2004) Supramolecular architectures. Mater Today 7:24–32. https://doi.org/10.1016/S1369-7021(04)00186-5
Blass BE (2015) Basic principles of drug discovery and development, 1st edn. Academic, New York
Viegas-Junior C, Danuello A, da Silva Bolzani V et al (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852. https://doi.org/10.2174/092986707781058805
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125–137. https://doi.org/10.1016/j.ejmech.2006.09.019
Zhuang C, Zhang W, Sheng C et al (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810. https://doi.org/10.1021/acs.chemrev.7b00020
Chavan BB, Gadekar AS, Mehta PP et al (2016) Synthesis and medicinal significance of chalcones—a review. Asian J Biomed Pharm Sci 2015(6):1–7
Singh P, Anand A, Kumar V (2014) Recent developments in biological activities of chalcones: a mini review. Eur J Med Chem 85:758–777. https://doi.org/10.1016/j.ejmech.2014.08.033
Wang HM, Zhang L, Liu J et al (2015) Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. Eur J Med Chem 92:439–448. https://doi.org/10.1016/j.ejmech.2015.01.007
Sivakumar PM, Prabhawathi V, Doble M (2010) Antibacterial activity and QSAR of chalcones against biofilm-producing bacteria isolated from marine waters. SAR QSAR Environ Res 21:247–263. https://doi.org/10.1080/10629361003771009
Patil CB, Mahajan SK, Katti SA (2009) Chalcone: aversatile molecule. J Pharm Sci Res 1:11–22. https://doi.org/10.1002/chin.201025251
Chimenti F, Fioravanti R, Bolasco A et al (2009) Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem 52:2818–2824. https://doi.org/10.1021/jm801590u
Engelbrecht I, Petzer JP, Petzer A (2018) Nitrocatechol derivatives of chalcone as inhibitors of monoamine oxidase and catechol-O-methyltransferase. Cent Nerv Syst Agents Med Chem 18:115–127. https://doi.org/10.2174/1871524918666180426125714
Rampa A, Montanari S, Pruccoli L et al (2017) Chalcone-based carbamates for Alzheimer’s disease treatment. Future Med Chem 9:749–764. https://doi.org/10.4155/fmc-2017-0029
Rampa A, Bartolini M, Pruccoli L et al (2018) Exploiting the chalcone scaffold to develop multifunctional agents for Alzheimer’s disease. Molecules 23:1902. https://doi.org/10.3390/molecules23081902
Rybka M, Mercader AG, Castro EA (2014) Predictive QSAR study of chalcone derivatives cytotoxicity activity against HT-29 human colon adenocarcinoma cell lines. Chemom Intell Lab Syst 132:18–29. https://doi.org/10.1016/j.chemolab.2013.12.005
Seo WD, Kim JH, Kang JE et al (2005) Sulfonamide chalcone as a new class of α-glucosidase inhibitors. Bioorganic Med Chem Lett 15:5514–5516. https://doi.org/10.1016/j.bmcl.2005.08.087
Seo WD, Ryu YB, Curtis-Long MJ et al (2010) Evaluation of anti-pigmentary effect of synthetic sulfonylamino chalcone. Eur J Med Chem 45:2010–2017. https://doi.org/10.1016/j.ejmech.2010.01.049
De Castro MRC, Aragão ÂQ, Da Silva CC et al (2016) Conformational variability in sulfonamide chalcone hybrids: crystal structure and cytotoxicity. J Braz Chem Soc 27:884–898. https://doi.org/10.5935/0103-5053.20150341
Remko M, Kožíšek J, Semanová J, Gregáň F (2010) Synthesis, crystal and molecular structure of two biologically active aromatic sulfonamides and their hydrochloride salts. J Mol Struct 973:18–26. https://doi.org/10.1016/j.molstruc.2010.03.013
Domínguez JN, León C, Rodrigues J et al (2005) Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Farmaco 60:307–311. https://doi.org/10.1016/j.farmac.2005.01.005
McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr Sect B Struct Sci 60:627–668. https://doi.org/10.1107/S0108768104020300
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32. https://doi.org/10.1039/B818330A
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun:3814. https://doi.org/10.1039/B704980C
Pereira DH, La PFA, Santiago RT et al (2016) New perspectives on the role of frontier molecular orbitals in the study of chemical reactivity: a review. Rev Virtual Química 8:425–453. https://doi.org/10.5935/1984-6835.20160032
Gilman JJ (1997) Chemical and physical “hardness”. Mater Res Innov 1:71–76. https://doi.org/10.1007/s100190050023
Sjoberg P, Politzer P (1990) Use of the electrostatic potential at the molecular surface. 3959–3961. https://doi.org/10.1021/j100373a017
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15. https://doi.org/10.1021/ar00109a003
Bader RFW (1989) Atoms in molecules in external fields. J Chem Phys 91:6989. https://doi.org/10.1063/1.457315
Bader RFW (2005) The quantum mechanical basis of conceptual chemistry. Monatsh Chem 136:819–854. https://doi.org/10.1007/s00706-005-0307-x
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928. https://doi.org/10.1021/cr00005a013
Kim JH, Ryu W, Shim H, Park H (2009) Development of new and selective Trypanosoma cruzi trans-sialidase inhibitors from sulfonamide chalcones and their derivatives. 701:2475–2479. https://doi.org/10.1002/cbic.200900108
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32:837–838. https://doi.org/10.1107/S0021889899006020
Dolomanov O V, Bourhis LJ, Gildea RJ, et al (2009) OLEX2 : a complete structure solution , refinement and analysis program. J Appl Crystal 42:339–341. https://doi.org/10.1107/S0021889808042726
Sheldrick GM (1990) SHELXS: Program for the solution of crystal structures. University of Göttingen, Göttingen
Muller P, Herbst-Irmer R, Spek AL et al (2006) Crystal structure refinement—a crystallographer’s guide to SHELXL, 1st edn. Oxford University Press, New York
Sheldrick GM (2014) Crystal structure refinement with SHELXL. Acta Crystallogr C C71: 3–8 . https://doi.org/10.1107/S20532296140242183
Macrae CF, Edgington PR, McCabe P et al (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457. https://doi.org/10.1107/S002188980600731X
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater 72:171–179. https://doi.org/10.1107/S2052520616003954
Mckinnon JJ, Mitchell AS, Spackman MA (1998) Visualising intermolecular interactions in crystals: naphthalene vs. terephthalic acid. Chem Commun 1998:2071–2072. https://doi.org/10.1039/a804691c
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Turner MJ, McKinnon JJ, Jayatilaka D, Spackman MA (2011) Visualisation and characterisation of voids in crystalline materials. CrystEngComm 13:1804–1813. https://doi.org/10.1039/C0CE00683A
Dalal J, Sinha N, Yadav H, Kumar B (2015) Structural, electrical, ferroelectric and mechanical properties with Hirshfeld surface analysis of novel NLO semiorganic sodium p-nitrophenolate dihydrate piezoelectric single crystal. RSC Adv 5:57735–57748. https://doi.org/10.1039/C5RA10501C
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4:378–392. https://doi.org/10.1039/B203191B
Wolff SK, Grimwood DJ, McKinnon JJ, et al (2012) Crystal Explorer 3.0. University of Western Australia, Perth
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision A.02. Gaussian Inc., Wallingford CT
Becke AD (1993) Densityfunctional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648. https://doi.org/10.1063/1.464913
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Sousa SF, Fernandes PA, Ramos MJ (2007) General performance of density functionals. J Phys Chem A 111:10439–10452 https://doi.org/10.1021/jp0734474
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474. https://doi.org/10.1103/PhysRevLett.55.2471
CPMD version 3.17.1: Copyright IBM (2012) https://www.mcs.anl.gov/~jlow/CPMD/manual-3.17.1.pdf
Santin LG, Toledo EM, Carvalho-Silva VH et al (2016) Methanol solvation effect on the proton rearrangement of curcumin’s enol forms: an ab initio molecular dynamics and electronic structure viewpoint. J Phys Chem C 120:19923–19931. https://doi.org/10.1021/acs.jpcc.6b02393
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41:7892–7895. https://doi.org/10.1103/PhysRevB.41.7892
Verlet L (1967) Computer “experiments” on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys Rev 159:98–103
Martyna GJ, Klein ML, Tuckerman M (1992) Nosé–Hoover chains: the canonical ensemble via continuous dynamics. J Chem Phys 97:2635–2643. https://doi.org/10.1063/1.463940
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
Nosé S (2002) A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 100:191–198. https://doi.org/10.1080/0026897011008910
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31:1695–1697. https://doi.org/10.1103/PhysRevA.31.1695
Oliveira BG, Araújo RCMU, Ramos MN (2010) A Topologia molecular qtaim e a descrição mecânico- quântica de ligações de hidrogênio e ligações de di-hidrogênio. Quim Nova 33:1155–1162. https://doi.org/10.1590/S0100-40422010000500029
Galdino B, Oliveira D, De CR et al (2009). Uma comprovação químico-quântica sobre a formação de ligações de hidrogênio e interações secundárias em sistemas heterocíclicos intermoleculares Introdução 1:167–182
Gatti C, Saunders VR, Roetti C (1994) Crystal field effects on the topological properties of the electron density in molecular crystals: the case of urea. J Chem Phys 101:10686–10696. https://doi.org/10.1063/1.467882
Koch U, Popelier PLA (1995) Characterization of C–H–O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754. https://doi.org/10.1021/j100024a016
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878
Bruno IJ, Cole JC, Kessler M et al (2004) Retrieval of crystallographically-derived molecular geometry information. J Chem Inf Comput Sci 44:2133–2144. https://doi.org/10.1021/ci049780b
Cepa MM, Tavares da Silva EJ, Correia da Silva G et al (2005) Structure–activity relationships of new A,D-ring modified steroids as aromatase Inhibitors: design, synthesis, and biological activity evaluation. J Med Chem 48:6379–6385. https://doi.org/10.1021/jm050129p
Sulpizio C, Roller A, Giester G, Rompel A (2016) Synthesis, structure, and antioxidant activity of methoxy- and hydroxyl-substituted 2′-aminochalcones. Monatsh Chem 147:1747–1757. https://doi.org/10.1007/s00706-016-1812-9
Szczesio M, Gołka J, Korona-Głowniak I (2018). Planarity of heteroaryldithiocarbazic acid derivatives showing tuberculostatic activity: structure–activity relationships research papers. 2:400–405. https://doi.org/10.1107/S205322961800284X
Szczesio M, Korona-Glowniak I, Gobis K (2018) Planarity of benzoyldithiocarbazate tuberculostatics. V. Antibacterial activities of diesters of benzoyldithiocarbazic acid. J Mol Struct 1167:127–133. https://doi.org/10.1016/j.molstruc.2018.04.072
Tu B, Liu Z, Chen Z et al (2015) Understanding the structure–activity relationship between quercetin and naringenin : in vitro. RSC Adv 5:106171–106181. https://doi.org/10.1039/C5RA22551E
Galabov B, Nikolova V, Ilieva S (2013) Does the molecular electrostatic potential reflect the effects of substituents in aromatic systems? Chmistry 19:5149–5155. https://doi.org/10.1002/chem.201204092
Koca M, Yildirim G, Kirilmis C, Karaboga F (2012) Density functional theory study on the identification of Pd(Me-Xanthate)2. Arab J Sci Eng 37:1283–1291. https://doi.org/10.1007/s13369-012-0251-0
Scrocco E, Tomasi J (1978) Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. Adv Quantum Chem 11:115–193. https://doi.org/10.1016/S0065-3276(08)60236-1
Demircioǧlu Z, Albayrak Ç, Büyükgüngör O (2014) Theoretical and experimental investigation of (E)-2-([3,4-dimethylphenyl) imino]methyl)-3-methoxyphenol: enol-keto tautomerism, spectroscopic properties, NLO, NBO and NPA analysis. J Mol Struct 1065–1066:210–222. https://doi.org/10.1016/j.molstruc.2014.02.062
Kılıç-Cıkla I, Güveli Ş, Yavuz M et al (2016) 5-Methyl-2-hydroxy-acetophenone-thiosemicarbazone and its nickel(II) complex: crystallographic, spectroscopic (IR, NMR and UV) and DFT studies. Polyhedron 105:104–114. https://doi.org/10.1016/j.poly.2015.12.021
Lu T, Chen F (2011) Multiwfn: a multifunctional wavefunction analyzer. https://doi.org/10.1002/jcc.22885
Silva López C, Olalla ], Faza N, et al. Ellipticity: a convenient tool to characterize electrocyclic reactions. Chemistry 11:1734–1738. https://doi.org/10.1002/chem.200401026
Cremer D, Kraka E, Nguyen-Dang TT et al (1983) Description of homoaromaticity in terms of electron distributions. J Am Chem Soc 105:5069–5075. https://doi.org/10.1021/ja00353a036
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support. Theoretical calculations were performed in the High-Performance Computing Center of Universidade Estadual de Goiás (UEG).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection VII Symposium on Electronic Structure and Molecular Dynamics – VII SeedMol
Electronic supplementary material
ESM 1
(DOCX 1171 kb)
Rights and permissions
About this article
Cite this article
Duarte, V.S., D’Oliveira, G.D.C., Custodio, J.M.F. et al. Experimental and molecular modeling study of a novel arylsulfonamide chalcone. J Mol Model 25, 208 (2019). https://doi.org/10.1007/s00894-019-4082-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4082-8