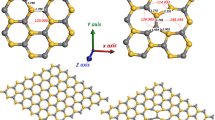
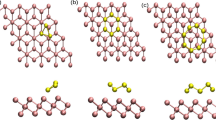
Abstract
Benzene, toluene, ethylbenzene, and xylenes are volatile hydrocarbons known as BTEX, which present concerns about environmental problems. Density functional theory (DFT) functionals were used for the BTEX gas phase adsorption on TiO2 (110) of rutile and (101) of anatase surfaces. Dispersion terms have shown the importance to treat weak interactions and were used to study these adsorptions using plane wave DFT calculations. All BTEX molecules have the same trend for the adsorption on rutile and anatase surfaces. The inclusion of dispersion terms has a significant contribution for the interaction energy. Density of states results suggest the hybridization between the d state of pentacoordinated titanium atoms (Ti5C) and carbon p states of benzene. The adsorption energy values indicate an effective interaction between the BTEX and surfaces, mainly due to the aromatic π interaction, which is present in all adsorbates. However, for p-xylene the methyl hydrogen directs the second major influence.

Charge difference showing the system with the smallest interaction and the one with the largest interaction







Similar content being viewed by others
References
Becher R, Hongslo JK, Jantunen MJ, Dybing E (1996) Environmental chemicals relevant for respiratory hypersensitivity: the indoor environment. Toxicol Lett 86:155–162. https://doi.org/10.1016/0378-4274(96)03685-5
Field RA, Goldstone ME, Lester JN, Perry R (1992) The sources and behaviour of tropospheric anthropogenic volatile hydrocarbons. Atmos Environ Part A 26:2983–2996. https://doi.org/10.1016/0960-1686(92)90290-2
Bertoni G, Ciuchini C, Pasini A, Tappa R (2002) Monitoring of ambient BTX at Monterotondo (Rome) and indoor-outdoor evaluation in school and domestic sites. J Environ Monit 4:903–909. https://doi.org/10.1039/B206959H
Cocheo V, Sacco P, Boaretto C, De Saeger E, Ballesta PP, Skov H, Goelen E, Gonzalez N, Caracena AB (2000) Urban benzene and population exposure. Nature 404:141–142
Sarigiannis DA, Karakitsios SP, Gotti A, Liakos IL, Katsoyiannis A (2011) Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ Int 37:743–765. https://doi.org/10.1016/j.envint.2011.01.005
Seinfeld JH, Spyros NP (2006) Atmospheric chemistry and physics: from air pollution to climate change, 2nd edn. Wiley, Hoboken
Gonçalves NS, Rettori D, Silva GMG, Noda LK (2018) Spectroscopic study of radical cation species formed on sulfated TiO2 upon benzene adsorption. Vib Spectrosc 99:80–85. https://doi.org/10.1016/j.vibspec.2018.08.012
Chen MS, Santra AK, Goodman DW (2004) Adsorption of benzene on a Mo(112)-c(2x2)- SiO4 surface. J Phys Chem B 108:17940–17945. https://doi.org/10.1021/jp047794e
Gomez-Romero P (2001) Hybrid organic-inorganic materials - in search of synergic activity. Adv Mater 13:163–174. https://doi.org/10.1002/1521-4095(200102)13:3<163::aid-adma163>3.3.co;2-l
Kang H, Lim S, Park N, Chun K-Y, Baik S (2010) Improving the sensitivity of carbon nanotube sensors by benzene functionalization. Sensors Actuators B Chem 147:316–321. https://doi.org/10.1016/j.snb.2010.03.028
Tautz FS (2007) Structure and bonding of large aromatic molecules on noble metal surfaces: the example of PTCDA. Prog Surf Sci 82:479–520. https://doi.org/10.1016/j.progsurf.2007.09.001
Zhou J, Dag S, Senanayake SD, Hathorn BC, Kalinin SV, Meunier V, Mullins DR, Overbury SH, Baddorf AP (2006) Adsorption, desorption, and dissociation of benzene on TiO2(110) and Pd/TiO2(110): experimental characterization and first-principles calculations. Phys Rev B 74:125318. https://doi.org/10.1103/PhysRevB.74.125318
Yu L, Ruan S, Xu X, Zou R, Hu J (2017) One-dimensional nanomaterial-assembled macroscopic membranes for water treatment. Nano Today 17:79–95. https://doi.org/10.1016/j.nantod.2017.10.012
Henderson MA (2013) Photooxidation and Photodesorption in the photochemistry of isobutene on TiO2(110). J Phys Chem C 117:14113–14124. https://doi.org/10.1021/jp404625c
Jwo C-S, Chang H, Kao M-J, Lin C-H (2007) Photodecomposition of volatile organic compounds using TiO2 nanoparticles. J Nanosci Nanotechnol 7:1947–1952. https://doi.org/10.1166/jnn.2007.746
Tanizaki T, Murakami Y, Hanada Y, Ishikawa S, Suzuki M, Shinohara R (2007) Titanium dioxide (TiO2)-assisted photocatalytic degradation of volatile organic compounds at ppb level. J Health Sci 53:514–519. https://doi.org/10.1248/jhs.53.514
Yu K, Huang L, Lou L-L, Chang Y, Dong Y, Wang H, Liu S (2015) Degradation of polycyclic aromatic hydrocarbons in crumb Tyre rubber catalysed by rutile TiO2 under UV irradiation. Environ Technol 36:1008–1015. https://doi.org/10.1080/09593330.2014.971883
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959. https://doi.org/10.1021/cr0500535
Diebold U (2003) The surface science of titanium dioxide. Surf Sci Rep 48:53–229. https://doi.org/10.1016/S0167-5729(02)00100-0
Fang W, Liu W, Guo X, Lu X, Lu L (2011) Theoretical investigation of CO adsorption on clean and hydroxylated TiO2-B (100) surfaces. J Phys Chem C 115:8622–8629. https://doi.org/10.1021/jp110825y
Mazheika AS, Bredow T, Matulis VE, Ivashkevich OA (2011) Theoretical study of adsorption of Ag clusters on the anatase TiO2(100) surface. J Phys Chem C 115:17368–17377. https://doi.org/10.1021/jp200575u
McGill PR, Muir JMR, Idriss H, Soehnel T (2013) Formamide adsorption over the TiO2 (110) surface: a theoretical study. RSC Adv 3:16829–16839. https://doi.org/10.1039/c3ra41286e
Mutombo P, Balazs N, Majzik Z, Berko A, Chab V (2012) Theoretical study of the adsorption of rhodium on a TiO2(110)-1 x 1 surface. Appl Surf Sci 258:4478–4482. https://doi.org/10.1016/j.apsusc.2012.01.011
Nieto-Lopez I, Hernandez-Garcia L, Bonilla-Cruz J, Sanchez M (2014) Theoretical study of absorption of 2,2,6,6-tetramethylpiperidine-1-oxoammonium cation (TEMPO) on TiO2(110) rutile surface. J Mol Model 20:2149. https://doi.org/10.1007/s00894-014-2149-0
Suzuki S, Tsuneda T, Hirao K (2012) A theoretical investigation on photocatalytic oxidation on the TiO2 surface. J Chem Phys 136:104701. https://doi.org/10.1063/1.3676261
Wanbayor R, Deak P, Frauenheim T, Ruangpornvisuti V (2011) First principles theoretical study of the hole-assisted conversion of CO to CO2 on the anatase TiO2(101) surface. J Chem Phys 134:104701. https://doi.org/10.1063/1.3562366
Wei Z, Zhang S, Pan Z, Liu Y (2011) Theoretical studies of arsenite adsorption and its oxidation mechanism on a perfect TiO2 anatase (1 0 1) surface. Appl Surf Sci 258:1192–1198. https://doi.org/10.1016/j.apsusc.2011.09.069
Zhao Z, Li Z, Zou Z (2012) A theoretical study of water adsorption and decomposition on the low-index stoichiometric Anatase TiO2 surfaces. J Phys Chem C 116:7430–7441. https://doi.org/10.1021/jp212407s
Bai J, Zhou B (2014) Titanium dioxide nanomaterials for sensor applications. Chem Rev 114:10131–10176. https://doi.org/10.1021/cr400625j
Diebold U (2003) Structure and properties of TiO2 surfaces: a brief review. Appl Phys A Mater Sci Process 76:681–687. https://doi.org/10.1007/s00339-002-2004-5
Herman GS, Sievers MR, Gao Y (2000) Structure determination of the two-domain (1 x 4) anatase TiO2(001) surface. Phys Rev Lett 84:3354–3357. https://doi.org/10.1103/PhysRevLett.84.3354
Lazzeri M, Vittadini A, Selloni A (2001) Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys Rev B 63:155409. https://doi.org/10.1103/PhysRevB.63.155409
Arrouvel C, Digne M, Breysse M, Toulhoat H, Raybaud P (2004) Effects of morphology on surface hydroxyl concentration: a DFT comparison of anatase–TiO2 and γ-alumina catalytic supports. J Catal 222:152–166. https://doi.org/10.1016/j.jcat.2003.10.016
Labat F, Baranek P, Adamo C (2008) Structural and electronic properties of selected rutile and anatase TiO2 surfaces: an ab initio investigation. J Chem Theory Comput 4:341–352. https://doi.org/10.1021/ct700221w
Perron H, Domain C, Roques J, Drot R, Simoni E, Catalette H (2007) Optimisation of accurate rutile TiO2 (110), (100), (101) and (001) surface models from periodic DFT calculations. Theor Chem Accounts 117:565–574. https://doi.org/10.1007/s00214-006-0189-y
Bennett RA, Stone P, Price NJ, Bowker M (1999) Two (1 x 2) reconstructions of TiO2(110): surface rearrangement and reactivity studied using elevated temperature scanning tunneling microscopy. Phys Rev Lett 82:3831–3834. https://doi.org/10.1103/PhysRevLett.82.3831
Diebold U, Anderson JF, Ng KO, Vanderbilt D (1996) Evidence for the tunneling site on transition-metal oxides: TiO2(110). Phys Rev Lett 77:1322–1325. https://doi.org/10.1103/PhysRevLett.77.1322
Hebenstreit W, Ruzycki N, Herman GS, Gao Y, Diebold U (2000) Scanning tunneling microscopy investigation of the TiO2 anatase (101) surface. Phys Rev B 62:R16334–R16336. https://doi.org/10.1103/PhysRevB.62.R16334
Hengerer R, Bolliger B, Erbudak M, Grätzel M (2000) Structure and stability of the anatase TiO2 (101) and (001) surfaces. Surf Sci 460:162–169 https://doi.org/10.1016/S0039-6028(00)00527-6
Hird B, Armstrong RA (1997) Ion scattering measurements of rutile TiO2(110)-(1 × 1) surface relaxation. Surf Sci 385:L1023–L1028. https://doi.org/10.1016/S0039-6028(97)00437-8
Lindsay R, Wander A, Ernst A, Montanari B, Thornton G, Harrison NM (2005) Revisiting the surface structure of TiO2(110): a quantitative low-energy electron diffraction study. Phys Rev Lett 94:246102. https://doi.org/10.1103/PhysRevLett.94.246102
Chuang Y-H, Hong G-B, Chang C-T (2014) Study on particulates and volatile organic compounds removal with TiO2 nonwoven filter prepared by electrospinning. J Air Waste Manage Assoc 64:738–742. https://doi.org/10.1080/10962247.2014.889614
Jeong J, Sekiguchi K, Lee W, Sakamoto K (2005) Photodegradation of gaseous volatile organic compounds (VOCs) using TiO2 photoirradiated by an ozone-producing UV lamp: decomposition characteristics, identification of by-products and water-soluble organic intermediates. J Photochem Photobiol A Chem 169:279–287. https://doi.org/10.1016/j.jphotochem.2004.07.014
Palau J, Colomer M, Penya-Roja JM, Martinez-Soria V (2012) Photodegradation of toluene, m-xylene, and n-butyl acetate and their mixtures over TiO2 catalyst on glass fibers. Ind Eng Chem Res 51:5986–5994. https://doi.org/10.1021/ie300357x
Zhang F, Zhu X, Ding J, Qi Z, Wang M, Sun S, Bao J, Gao C (2014) Mechanism study of photocatalytic degradation of gaseous toluene on TiO2 with weak-bond adsorption analysis using in situ far infrared spectroscopy. Catal Lett 144:995–1000. https://doi.org/10.1007/s10562-014-1213-9
Farias SAS, Longo E, Gargano R, Martins JBL (2013) CO2 adsorption on polar surfaces of ZnO. J Mol Model 19:2069–2078. https://doi.org/10.1007/s00894-012-1636-4
Sorescu DC, Al-Saidi WA, Jordan KD (2011) CO2 adsorption on TiO2(101) anatase: a dispersion-corrected density functional theory study. J Chem Phys 135:124701. https://doi.org/10.1063/1.3638181
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561. https://doi.org/10.1016/0022-3093(95)00355-X
Kresse G, Hafner J (1994) Ab-initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys Rev B 49:14251–14269. https://doi.org/10.1103/PhysRevB.49.14251
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces - applications of the generalized approximation for exchange and correlation. Phys Rev B 46:6671–6687. https://doi.org/10.1103/PhysRevB.46.6671
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Erratum: atoms, molecules, solids, and surfaces - applications of the generalized approximation for exchange and correlation. Phys Rev B 48:4978–4978. https://doi.org/10.1103/PhysRevB.48.4978.2
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Perdew JP, Burke K, Ernzerhof M (1997) Erratum: generalized gradient approximation made simple. Phys Rev Lett 78:1396–1396. https://doi.org/10.1103/PhysRevLett.78.1396
Mattsson AE, Armiento R, Schultz PA, Mattsson TR (2006) Nonequivalence of the generalized gradient approximations PBE and PW91. Phys Rev B 73:195123. https://doi.org/10.1103/PhysRevB.73.195123
Blochl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979. https://doi.org/10.1103/PhysRevB.50.17953
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775. https://doi.org/10.1103/PhysRevB.59.1758
Burdett JK, Hughbanks T, Miller GJ, Richardson JW, Smith JV (1987) Structural-electronic relationships in inorganic solids: powder neutron diffraction studies of the rutile and anatase polymorphs of titanium dioxide at 15 and 295 K. J Am Chem Soc 109:3639–3646. https://doi.org/10.1021/ja00246a021
Horn M, Schwerdt C, Meagher EP (1972) Refinement of structure of anatase at several temperatures. Z Krist 136:273–281. https://doi.org/10.1524/zkri.1972.136.3-4.273
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Hasnip PJ, Refson K, Probert MIJ, Yates JR, Clark SJ, Pickard CJ (2014) Density functional theory in the solid state. Philos Transact A Math Phys Eng Sci 372:20130270. https://doi.org/10.1098/rsta.2013.0270
Bates SP, Kresse G, Gillan MJ (1997) A systematic study of the surface energetics and structure of TiO2(110) by first-principles calculations. Surf Sci 385:386–394. https://doi.org/10.1016/S0039-6028(97)00265-3
Lindan PJD, Harrison NM, Gillan MJ, White JA (1997) First-principles spin-polarized calculations on the reduced and reconstructed TiO2 (110) surface. Phys Rev B 55:15919–15927. https://doi.org/10.1103/PhysRevB.55.15919
Liu H, Wang X, Pan C, Liew KM (2012) First-principles study of formaldehyde adsorption on TiO2 rutile (110) and Anatase (001) surfaces. J Phys Chem C 116:8044–8053. https://doi.org/10.1021/jp210465u
Ma J-G, Zhang C-R, Gong J-J, Yang B, Zhang H-M, Wang W, Wu Y-Z, Chen Y-H, Chen H-S (2014) The adsorption of alpha-cyanoacrylic acid on anatase TiO2 (101) and (001) surfaces: a density functional theory study. J Chem Phys 141:234705. https://doi.org/10.1063/1.4903790
Neto ARR, Alves HWL (2010) Adsorption of benzene over the rutile TiO2 (110) surfaces: a theoretical study. Phys Status Solidi C 7:308–311. https://doi.org/10.1002/pssc.200982434
Liu W, Carrasco J, Santra B, Michaelides A, Scheffler M, Tkatchenko A (2012) Benzene adsorbed on metals: concerted effect of covalency and van der Waals bonding. Phys Rev B 86:245405. https://doi.org/10.1103/PhysRevB.86.245405
Nagao M, Suda Y (1989) Adsorption of benzene, toluene, and chlorobenzene on titanium dioxide. Langmuir 5:42–47. https://doi.org/10.1021/la00085a009
Reiss S, Krumm H, Niklewski A, Staemmler V, Woll C (2002) The adsorption of acenes on rutile TiO2(110): a multi-technique investigation. J Chem Phys 116:7704–7713. https://doi.org/10.1063/1.1460855
Mabrook M, Hawkins P (2002) Benzene sensing using thin films of titanium dioxide operating at room temperature. Sensors 2:374–382. https://doi.org/10.3390/s20900374
Carrasco J, Liu W, Michaelides A, Tkatchenko A (2014) Insight into the description of van der Waals forces for benzene adsorption on transition metal (111) surfaces. J Chem Phys 140:084704. https://doi.org/10.1063/1.4866175
Acknowledgments
The authors are indebted to the financial support of FAPDF, CNPq, and CAPES and the computational support of CENAPAD/SP and UnB/FINEP Institute of Chemistry Computational Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection VII Symposium on Electronic Structure and Molecular Dynamics – VII SeedMol
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
dos Reis Vargas, M., de Castro, E.A.S., Politi, J.R.d.S. et al. BTEX adsorption on TiO2 anatase and rutile surfaces: DFT functionals. J Mol Model 25, 137 (2019). https://doi.org/10.1007/s00894-019-4027-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4027-2