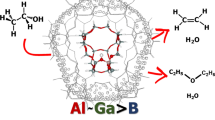
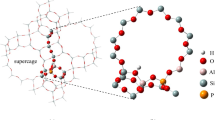
Abstract
The search for renewable raw materials less harmful to the environment, such as methanol, ethanol, 1-propanol, and 1-butanol has become attractive. These products are obtained more rapidly and efficiently by specific solid catalysts, mainly the zeolites. The Brønsted acid sites distributed over the sinusoidal and the straight channels are important for the alcohol dehydration reaction that produces widely used chemicals. Therefore, the ONIOM method was used to study methanol, ethanol, propanol, and butanol adsorption in H-ZSM-5 zeolite. PM6 and DFT levels were used for the high layer ONIOM, while the low layer was calculated using the UFF force field. DFT was calculated using the B3LYP global hybrid GGA, M06-2X hybrid meta-GGA, and the hybrid range separated ωB97X-D functionals at 6–31+G(d) basis set. The high layer ONIOM was completely relaxed. The binding energy shows dependence on the relaxed tetrahedra and position of acid site. The Si/Al ratio was also studied.

HOMO orbital of adsorbed alcohols showing the main contribution of zeolite for small alcohols




Similar content being viewed by others
References
Ramasamy KK, Wang Y (2013) Catalyst activity comparison of alcohols over zeolites. J Energy Chem 22:65–71. https://doi.org/10.1016/S2095-4956(13)60008-X
Jae J, Tompsett GA, Foster AJ, Hammond KD, Auerbach SM, Lobo RF, Huber GW (2011) Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J Catal 279:257–268. https://doi.org/10.1016/j.jcat.2011.01.019
Bjørgen M, Svelle S, Joensen F, Nerlov J, Kolboe S, Bonino F, Palumbo L, Bordiga S, Olsbye U (2007) Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: on the origin of the olefinic species. J Catal 249:195–207. https://doi.org/10.1016/j.jcat.2007.04.006
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89. https://doi.org/10.1038/nature06450
Deng R, Yao B, Li YF, Li BH, Zhang ZZ, Zhao HF, Zhang JY, Zhao DX, Shen DZ, Fan XW, Yang LL, Zhao QX (2009) Surface morphology, structural and optical properties of polar and non-polar ZnO thin films: a comparative study. J Cryst Growth 311:4398–4401. https://doi.org/10.1016/j.jcrysgro.2009.07.043
Ramasamy KK, Zhang H, Sun J, Wang Y (2014) Conversion of ethanol to hydrocarbons on hierarchical HZSM-5 zeolites. Catal Today 238:103–110. https://doi.org/10.1016/j.cattod.2014.01.037
Ghorbanpour A, Rimer JD, Grabow LC (2016) Computational assessment of the dominant factors governing the mechanism of methanol dehydration over H-ZSM-5 with heterogeneous aluminum distribution. ACS Catal 6:2287–2298. https://doi.org/10.1021/acscatal.5b02367
Breck DW (1964) Crystalline molecular sieves. J Chem Educ 41:678–689. https://doi.org/10.1021/ed041p678
Naber JE, de Jong KP, Stork WHJ, Kuipers HPCE, Post MFM (1994) Industrial applications of zeolite catalysis. Stud Surf Sci Catal 84:2197–2219. https://doi.org/10.1016/S0167-2991(08)63783-0
Song Z, Takahashi A, Mimura N, Fujitani T (2009) Production of propylene from ethanol over ZSM-5 zeolites. Catal Lett 131:364–369. https://doi.org/10.1007/s10562-009-0071-3
Roque-Malherbe R (2001) Handbook of surfaces and interfaces of materials: zeolites. Handb Surf Interface Mater 2:495–522. https://doi.org/10.1016/B978-012513910-6/50069-4
Auerbach SM, Carrado KA, Dutta PK (2003) Handbook of zeolite science and technology. CRC, Boca Raton
Bein T (1996) Synthesis and applications of molecular sieve layers and membranes. Chem Mater 8:1636–1653. https://doi.org/10.1021/cm960148a
Woolery GL, Kuehl GH (1997) On the nature of framework Brønsted and Lewis acid sites in ZSM-5. Zeolites 19:288–296. https://doi.org/10.1016/S0144-2449(97)00086-9
Wu W, Weitz E (2014) Modification of acid sites in ZSM-5 by ion-exchange: an in-situ FTIR study. Appl Surf Sci 316:405–415. https://doi.org/10.1016/j.apsusc.2014.07.194
Gao Y, Zheng B, Wu G, Ma F, Liu C (2016) Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv 6:83581–83588. https://doi.org/10.1039/c6ra17084f
Zhang J, Qian W, Kong C, Wei F (2015) Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst. ACS Catal 5:2982–2988. https://doi.org/10.1021/acscatal.5b00192
Decolatti HP, Dalla Costa BO, Querini CA (2015) Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with NaOH. Microporous Mesoporous Mater 204:180–189. https://doi.org/10.1016/j.micromeso.2014.11.014
Busca G (2017) Acidity and basicity of zeolites: a fundamental approach. Microporous Mesoporous Mater 254:3–16. https://doi.org/10.1016/j.micromeso.2017.04.007
Lercher JA, Jentys A, Brait A (2008) Catalytic test reactions for probing the acidity and basicity of zeolites. In: Acidity and Basicity. Molecular Sieves. Springer, Berlin, pp 153–212
Chang CD, Silvestri AJ (1977) The conversion of methanol and other O-compounds to hydrocarbons over zeolit catalysts. J Catal 47:249–259. https://doi.org/10.1016/0021-9517(77)90172-5
Olson DH, Kokotailo GT, Lawton SL, Meier WM (1981) Crystal structure and structure-related properties of ZSM-5. J Phys Chem 85:2238–2243. https://doi.org/10.1021/j150615a020
Van Koningsveld H (1990) High-temperature (350 K) orthorhombic framework structure of zeolite H-ZSM-5. Acta Crystallogr Sect B 46:731–735. https://doi.org/10.1107/S0108768190007522
van Koningsveld H, Jansen JC, van Bekkum H (1994) The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 10:235–242. https://doi.org/10.1016/0144-2449(94)90134-1
Stöcker M (1999) Methanol-to-hydrocarbons: catalytic materials and their behavior. Microporous Mesoporous Mater 29:3–48. https://doi.org/10.1016/S1387-1811(98)00319-9
Bjørgen M, Joensen F, Spangsberg Holm M, Olsbye U, Lillerud KP, Svelle S (2008) Methanol to gasoline over zeolite H-ZSM-5: improved catalyst performance by treatment with NaOH. Appl Catal A Gen 345:43–50. https://doi.org/10.1016/j.apcata.2008.04.020
Haag S, Hanebuth M, Mabande GTP, Avhale A, Schwieger W, Dittmeyer R (2006) On the use of a catalytic H-ZSM-5 membrane for xylene isomerization. Microporous Mesoporous Mater 96:168–176. https://doi.org/10.1016/j.micromeso.2006.06.032
Buchanan JS, Santiesteban JG, Haag WO (1996) Mechanistic considerations in acid-catalyzed cracking of olefins. J Catal 158:279–287. https://doi.org/10.1006/jcat.1996.0027
Tanabe K, Hölderich F (1999) Industrial application of solid acid–base catalysts. Appl Catal A Gen 181:399–434. https://doi.org/10.1016/S0926-860X(98)00397-4
Bryant D, Kranich WL (1967) Dehydration of alcohols over zeolite catalysts. J Catal 8:8–13. https://doi.org/10.1016/0021-9517(67)90275-8
West RM, Braden DJ, Dumesic JA (2009) Dehydration of butanol to butene over solid acid catalysts in high water environments. J Catal 262:134–143. https://doi.org/10.1016/j.jcat.2008.12.009
Takahashi A, Xia W, Wu Q, Furukawa T, Nakamura I, Shimada H, Fujitani T (2013) Difference between the mechanisms of propylene production from methanol and ethanol over ZSM-5 catalysts. Appl Catal A Gen 467:380–385. https://doi.org/10.1016/j.apcata.2013.07.064
Galli E, Vezzalini G, Quartieri S, Alberti A, Franzini M (1997) Mutinaite, a new zeolite from Antarctica: the natural counterpart of ZSM-5. Zeolites 19:318–322. https://doi.org/10.1016/S0144-2449(97)00100-0
Ohlin L, Bazin P, Thibault-Starzyk F, Hedlund J, Grahn M (2013) Adsorption of CO2, CH4, and H2O in zeolite ZSM-5 studied using in situ ATR-FTIR spectroscopy. J Phys Chem C 117:16972–16982. https://doi.org/10.1021/jp4037183
Fan D, Dai D-J, Wu H-S (2013) Ethylene formation by catalytic dehydration of ethanol with industrial considerations. Materials (Basel) 6:101–115. https://doi.org/10.3390/ma6010101
Maihom T, Khongpracha P, Sirijaraensre J, Limtrakul J (2013) Mechanistic studies on the transformation of ethanol into ethene over Fe-ZSM-5 zeolite. ChemPhysChem 14:101–107. https://doi.org/10.1002/cphc.201200786
Jiang S, Hwang JS, Jin T, Cai T, Cho W, Baek YS, Park SE (2004) Dehydration of methanol to dimethyl ether over ZSM-5 zeolite. Bull Kor Chem Soc 25:185–189. https://doi.org/10.5012/bkcs.2004.25.2.185
Lesthaeghe D, VanderMynsbrugge J, Vandichel M, Waroquier M, VanSpeybroeck V (2011) Full theoretical cycle for both ethene and propene formation during methanol-to-olefin conversion in H-ZSM-5. ChemCatChem 3:208–212. https://doi.org/10.1002/cctc.201000286
Makarova MA, Paukshtis EA, Thomas JM, Williams C, Zamaraev KI (1994) Dehydration of n-butanol on zeolite H-ZSM-5 and amorphous aluminosilicate: detailed mechanistic study and the effect of pore confinement. J Catal 149:36–51. https://doi.org/10.1006/jcat.1994.1270
Huang Y, Dong X, Li M, Yu Y, Gao J, Zheng Y, Fitzgerald GB, de Joannis J, Tang Y, Wachs IE, Podkolzin SG, Huang Y, Dong X, Li M, Zhang M, Yu Y (2015) A density functional theory study on ethylene formation and conversion over P modified ZSM-5. Catal Sci Technol 5:1093–1105. https://doi.org/10.1039/C4CY01205D
Alexopoulos K, Lee MS, Liu Y, Zhi Y, Liu Y, Reyniers MF, Marin GB, Glezakou VA, Rousseau R, Lercher JA (2016) Anharmonicity and confinement in zeolites: structure, spectroscopy, and adsorption free energy of ethanol in H-ZSM-5. J Phys Chem C 120:7172–7182. https://doi.org/10.1021/acs.jpcc.6b00923
Nguyen CM, Reyniers M-F, Marin GB (2010) Theoretical study of the adsorption of C1–C4 primary alcohols in H-ZSM-5. Phys Chem Chem Phys 12:9481–9493. https://doi.org/10.1039/c000503g
Svensson M, Humbel S, Froese RDJ, Matsubara T, Sieber S, Morokuma K (1996) ONIOM: a multilayered integrated MO + MM method for geometry optimizations and single point energy predictions. A test for Diels−Alder reactions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J Phys Chem 100:19357–19363. https://doi.org/10.1021/jp962071j
Chung LW, Hirao H, Li X, Morokuma K (2012) The ONIOM method: its foundation and applications to metalloenzymes and photobiology. Wiley Interdiscip Rev Comput Mol Sci 2:327–350
Chung LW, Sameera WMC, Ramozzi R, Page AJ, Hatanaka M, Petrova GP, Harris TV, Li X, Ke Z, Liu F, Li HB, Ding L, Morokuma K (2015) The ONIOM method and its applications. Chem Rev 115:5678–5796
Martins JBL, Taft CA, Longo E, De Castro EAS, Da Cunha WF, Politi JRS, Gargano R (2012) ONIOM study of dissociated hydrogen and water on ZnO surface. Int J Quantum Chem 112:3223–3227. https://doi.org/10.1002/qua.24147
Guo YH, Pu M, Chen BH, Cao F (2013) Theoretical study on the cracking reaction catalyzed by a solid acid with zeolitic structure: the catalytic cracking of 1-hexene on the surface of H-ZSM-5. Appl Catal A Gen 455:65–70. https://doi.org/10.1016/j.apcata.2013.01.013
Soltanali S, Halladj R, Ektefa F (2015) A DFT exploration of the adsorption of methanol on the Brönsted acid site of nanostructured H-ZSM-5 catalyst: probing the effect of cluster size on the O–H···O hydrogen bond based on NMR and NQR parameters. J Clust Sci 26:565–579. https://doi.org/10.1007/s10876-014-0839-5
Schmidt W, Wilczok U, Weidenthaler C, Medenbach O, Goddard R, Buth G, Cepak A (2007) Preparation and morphology of pyramidal MFI single-crystal segments. J Phys Chem B 111:13538–13543. https://doi.org/10.1021/jp075934p
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M et al (2009) Gaussian 09, revision D.01. Gaussian, Inc, Wallingford
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213. https://doi.org/10.1007/s00894-007-0233-4
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Rappe AK, Casewit CJ, Colwell KS, Goddard WA, Skiff WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035. https://doi.org/10.1021/ja00051a040
Gomes GJ, Zalazar MF, Lindino CA, Scremin FR, Bittencourt PRS, Costa MB, Peruchena NM (2017) Adsorption of acetic acid and methanol on H-Beta zeolite: an experimental and theoretical study. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2017.06.008
Maihom T, Boekfa B, Sirijaraensre J, Nanok T, Probst M, Limtrakul J (2009) Reaction mechanisms of the methylation of Ethene with methanol and dimethyl ether over H-ZSM-5: an ONIOM study. J Phys Chem C 113:6654–6662. https://doi.org/10.1021/jp809746a
Patet RE, Caratzoulas S, Vlachos DG (2016) Adsorption in zeolites using mechanically embedded ONIOM clusters. Phys Chem Chem Phys 18:26094–26106. https://doi.org/10.1039/c6cp03266d
Scaranto J, Mallia G, Harrison NM (2011) An efficient method for computing the binding energy of an adsorbed molecule within a periodic approach. The application to vinyl fluoride at rutile TiO2(1 1 0) surface. Comput Mater Sci 50:2080–2086. https://doi.org/10.1016/j.commatsci.2011.02.011
Hunger M, Horvath T (1996) Adsorption of methanol on Bronsted acid sites in zeolite H-ZSM-5 investigated by multinuclear solid-state NMR spectroscopy. J Am Chem Soc 118:12302–12308. https://doi.org/10.1021/ja962425k
Hunger B, Matysik S, Heuchel M, Einicke W-D (1997) Adsorption of methanol on ZSM-5 zeolites. Langmuir 13:6249–6254. https://doi.org/10.1021/la970615i
Pope CG (1993) Adsorption of methanol and related molecules. J Chem Soc Faraday Trans 89:1139–1141. https://doi.org/10.1039/FT9938901139
Messow U, Quitzsch K, Herden H (1984) Heats of immersion of ZSM-5 zeolite in n-alkanes, 1-alkenes and 1-alcohols at 30°C. Zeolites 4:255–258. https://doi.org/10.1016/0144-2449(84)90034-4
Lee C-C, Gorte RJ, Farneth WE (1997) Calorimetric study of alcohol and nitrile adsorption complexes in H-ZSM-5. J Phys Chem B 101:3811–3817. https://doi.org/10.1021/jp970711s
Vayssilov GN, Lercher JA, Rösch N (2000) Interaction of methanol with alkali metal exchanged molecular sieves. 2. Density functional study. J Phys Chem B 104:8614–8623. https://doi.org/10.1021/jp000195x
Shah R, Gale JD, Payne MC (1996) Methanol adsorption in zeolites - a first-principles study. J Phys Chem 100:11688–11697. https://doi.org/10.1021/jp960365z
Yuan S, Wang J, Li Y, Jiao H (2003) Density functional investigations into the adsorption of methanol on Isomorphously substituted ZSM-5. J Nat Gas Chem 12:93–97
Piccini G, Alessio M, Sauer J (2018) Ab initio study of methanol and ethanol adsorption on Brønsted sites in zeolite H-MFI. Phys Chem Chem Phys 20:19964–19970. https://doi.org/10.1039/C8CP03632B
Nguyen CM, Reyniers MF, Marin GB (2015) Adsorption thermodynamics of C1-C4 alcohols in H-FAU, H-MOR, H-ZSM-5, and H-ZSM-22. J Catal 322:91–103. https://doi.org/10.1016/j.jcat.2014.11.013
Milestone NB, Bibby DM (1984) Adsorption of alcohols from aqueous solution by ZSM-5. J Chem Technol Biotechnol Chem Technol 34:73–79. https://doi.org/10.1002/jctb.5040340205
Aronson MT, Gorte RJ, Farneth WE (1987) An infrared spectroscopy study of simple alcohols adsorbed on H-ZSM-5. J Catal 105:455–468. https://doi.org/10.1016/0021-9517(87)90073-X
Mirth G, Lercher JA, Anderson MW, Klinowski J (1990) Adsorption complexes of methanol on zeolite ZSM-5. J Chem Soc Faraday Trans. https://doi.org/10.1039/FT9908603039
Ison A, Gorte RJ (1984) The adsorption of methanol and water on H-ZSM-5. J Catal 89:150–158. https://doi.org/10.1016/0021-9517(84)90289-6
Van der Borght K, Batchu R, Galvita VV, Alexopoulos K, Reyniers MF, Thybaut JW, Marin GB (2016) Insights into the reaction mechanism of ethanol conversion into hydrocarbons on H-ZSM-5. Angew Chem Int Ed 55:12817–12821. https://doi.org/10.1002/anie.201607230
Xin H, Li X, Fang Y, Yi X, Hu W, Chu Y, Zhang F, Zheng A, Zhang H, Li X (2014) Catalytic dehydration of ethanol over post-treated ZSM-5 zeolites. J Catal 312:204–215. https://doi.org/10.1016/j.jcat.2014.02.003
Dubinin MM, Rakhmatkariev GU, Isirikyan AA (1989) Heats of adsorption of methanol and ethanol on high-silicon ZSM-5 zeolite. Russ Chem Bull 38:2419–2421
Dahms F, Costard R, Pines E, Fingerhut BP, Nibbering ETJ, Elsaesser T (2016) The hydrated excess proton in the Zundel cation H5O2+: the role of ultrafast solvent fluctuations. Angew Chem Int Ed 55:10600–10605. https://doi.org/10.1002/anie.201602523
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76. https://doi.org/10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
Barone G, Casella G, Giuffrida S, Duca D (2007) {H-ZSM}-5 modified zeolite: quantum chemical models of acidic sites. J Phys Chem C 111:13033–13043. https://doi.org/10.1021/jp066652c
Acknowledgments
The authors acknowledge the financial support of CAPES and CNPq, FAPDF and also the helpful discussion with the colleagues J. A. Dias and S. C. L. Dias. Computational resources were provided at UnB – FINEP Computational Center of Chemistry Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection VII Symposium on Electronic Structure and Molecular Dynamics – VII SeedMol
Rights and permissions
About this article
Cite this article
Costa, R.J., Castro, E.A.S., Politi, J.R.S. et al. Methanol, ethanol, propanol, and butanol adsorption on H-ZSM-5 zeolite: an ONIOM study. J Mol Model 25, 34 (2019). https://doi.org/10.1007/s00894-018-3894-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3894-2