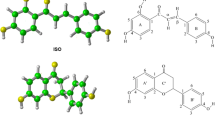
Abstract
Free radicals can be scavenged from biological systems by genistein, daidzein, and their methyl derivatives through hydrogen atom transfer (HAT), single-electron transfer (SET), and sequential proton-loss electron-transfer (SPLET) mechanisms. Reactions between these derivatives and the free radicals OH., OCH3., and NO2. via the HAT mechanism in the gas phase were studied using the transition state theory within the framework of DFT. Solvation of all the species and complexes involved in the HAT reactions in aqueous media was treated by performing single point energy calculations using the polarizable continuum model (PCM). The SET and SPLET mechanisms for the above reactions were also considered by applying the Marcus theory of electron transfer, and were found to be quite sensitive to geometry and solvation. Therefore, the geometries of all the species involved in the SET and SPLET mechanisms were fully optimized in aqueous media. The calculated barrier energies and rate constants of the HAT-based scavenging reactions showed that the OH group of the B ring in genistein, daidzein, and their methyl derivatives plays a major role in the scavenging of free radicals, and the role of this OH group in the HAT-based free-radical scavenging decreases in the following order: OH. > OCH3. > NO2.. The SPLET mechanism was found to be an important mechanism in these free-radical scavenging reactions, whereas the SET mechanism was not important in this context.







Similar content being viewed by others
References
Fridovich I (1978) Science 201:875–880
Halliwell B, Gutteridge JM (1984) Biochem J 219:1–14
Darley-Usmar V, Halliwell B (1996) Pharm Res 13:649–662
Yarkony DR, Schaefer HF, Rothenberg S (1974) J Am Chem Soc 96:656–659
Niles JC, Wishnok JS, Tannenbaum SR (2006) Nitric Oxide 14:109–121
Sodum RS, Fiala ES (2001) Chem Res Toxicol 14:438–450
Pavlovic R, Santaniello E (2007) J Pharm Pharmacol 59:1687–1695
Pogozelski WK, Tullius TD (1998) Chem Rev 98:1089–1108
Sonntag V (2006) Free-radical-induced DNA damage and its repair: a chemical perspective. Springer, Berlin
Greenberg MM (2007) Org Biomol Chem 5:18–30
Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S (1999) Mutat Res 424:9–21
Burrows CJ, Muller JG (1998) Chem Rev 98:1109–1152
Halliwell B (1999) Mutat Res 443:37–52
Simons J (2006) Acc Chem Res 39:772–779
Mishina Y, Duguid EM, He C (2006) Chem Rev 106:215–232
Tiwari MK, Mishra PC (2013) J Mol Model 19:5445–5456
Tiwari MK, Mishra PC (2016) J Chem Sci 128:1199–1210
Tiwari MK, Mishra PC (2016) RSC Adv 6:86650–86662
Tiwari MK, Jena NR, Mishra PC (2018) J Chem Sci 130:105–121
Gulçin I (2012) Arch Toxicol 86:345–391
Craft BD, Kerrihard AL, Amarowicz R, Pegg RB (2012) Compr Rev Food Sci Food Saf 11:148–173
Setchell KD (1998) Am J Clin Nutr 68:1333S–1346S
Ososki AL, Kennelly EJ (2003) Phytother Res 17:845–869
Anderson JJB, Anthony M, Messina M, Garner SC (1999) Nutr Res Rev 12:75–116
Adlercreutz H, Mazur W, Bartels P, Elomaa VV, Watanabe S, Wahala K, Landstrom M, Lundin E, Bergh A, Damber JE, Aman P, Widmark A, Johansson A, Zhang JX, Hallmans GJ (2000) J Nutr 130:658–659
de Lemos ML (2001) Ann Pharmacother 35:1118–1121
Hao A, Zhang Y, Zhang H, Liu Y, Xu X (2013) J Pharm Pharmacol 7:199
Davis JN, Kucuk O, Sarkar FH (1999) Nutr Cancer 35:167–174
Davis JN, Kucuk O, Djuric Z, Sarkar FH (2001) Free Radic Biol Med 30:1293–1302
Kurzer MS, Xu X (1997) Annu Rev Nutr 17:353–381
Yamashita Y, Kawada SZ, Nakaro H (1990) Biochem Pharmacol 39:737–744
Okura A, Arakawa H, Oka H, Yoshinari T, Monnden Y (1988) Biochem Biophys Res Commun 157:183–189
Setchell KDR, Cassidy A (1999) J Nutr 129:758S–767S
Agnihotri N, Mishra PC (2011) J Phys Chem A 115:14221–14232
Wright JS, Johnson ER, DiLabio GA (2001) J Am Chem Soc 123:1173–1183
Lee SH, Baek K, Lee JE, Kim BG (2016) Biotechnol Bioeng 113:735–743
Chiang CM, Wang DS, Chang TS (2016) Molecules 21:1723–1732
Chiang CM, Ding HY, Tsai YT, Chang TS (2015) Int J Mol Sci 16:27816–27823
Marcus RA (1964) Annu Rev Phys Chem 15:155–196
Marcus RA (1993) Rev Mod Phys 65:599–610
Marcus RA (1997) Pure Appl Chem 69:13–30
Litwinienko G, Ingold KU (2003) J Org Chem 68:3433–3438
Litwinienko G, Ingold KU (2004) J Org Chem 69:5888–5896
Litwinienko G, Ingold KU (2005) J Org Chem 70:8982–8990
Singh H, Singh S, Srivastava A, Tandon P, Bharti P, Kumar S, Maurya R (2014) Spectrochim Acta A 120:405–415
Zhao Y, Truhlar DG (2006) J Phys Chem A 110:5121–5129
Zhao Y, Truhlar DG (2008) J Chem Theory Comput 4:1849–1868
Hariharan PC, Pople JA (1972) Chem Phys Lett 16:217–219
Miertus S, Tomasi J (1982) Chem Phys 65:239–245
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Nelsen SF, Blackstock SC, Kim Y (1987) J Am Chem Soc 109:677–682
Nelsen SF, Weaver MN, Luo Y, Pladziewicz JR, Ausman LK, Jentzsch TL, O’Konek JJ (2006) J Phys Chem A 110:11665–11676
Laidler KJ (2004) In: Chemical kinetics, 3rd edn. Pearson Education (Singapore) Pte Ltd. (Indian Branch), Patparganj
Skodje RT, Truhlar DG (1981) J Phys Chem 34:624–628
Frisch MJ, Trucks GW, Schlegel HB, et al (2009) Gaussian 09, revision D.01. Gaussian Inc., Wallingford
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem Inc., Shawnee Mission
Lengyel J, Rimarcik J, Vaganek A, Klein E (2013) Phys Chem Chem Phys 15:10895–10903
Zhang HY, Wang LF, Sun YM (2003) Bioorg Med Chem Lett 13:909
Zielonka J, Gebicki J, Grynkiewicz G (2003) Free Radic Biol Med 35:958
Acknowledgements
One of the authors (PCM) is thankful to the National Academy of Sciences, India (NASI) for the award of a Senior Scientist Fellowship along with the related financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
ESM 1
(DOCX 8999 kb)
Rights and permissions
About this article
Cite this article
Tiwari, M.K., Mishra, P.C. Scavenging of hydroxyl, methoxy, and nitrogen dioxide free radicals by some methylated isoflavones. J Mol Model 24, 287 (2018). https://doi.org/10.1007/s00894-018-3805-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3805-6