Abstract
The efficient transport of a drug molecule until its target cell constitutes a significant challenge for delivery processes. To achieve such objectives, solid nanocapsules that protect the immune system during the transport should be developed and controlled at the nanoscale level. From this point of view, nanostructures based on graphene sheets could present some promising properties due to their ultimate size and dimension. In this work, we present theoretical results using DFT calculations, dealing with a graphene-based delivery system. Indeed, we demonstrate the stability of the gemcitabine anticancer molecule when it is encapsulated into two concave graphene sheets organized as a nest. Quantum calculations showed that the most stable state is located inside the nest, which is then formed by two layers distanced 6 Å from each other. For all the optimized systems, we focused on the dependence of the interaction energy on the molecule displacements during its entrance in the graphene nest and its exit from it. We also analyzed their consequence on the local morphological and electronic charge properties.

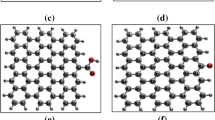
Adsorption energy (in eV) of gemcitabine drug during its encapsulation inside the nest of grapheneand its release from it







Similar content being viewed by others
References
Kuhlmann K, De Castro S, Wesseling J, Ten Kate F, Offerhaus G, Busch O, Van Gulik T, Obertop H, Gouma D (2004) Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer 40:549–558
Pollera CF, Ceribelli A, Crecco M, Oliva C (1997) Prolonged infusion gemcitabine: a clinical phase I study at low- (300 mg/m2) and high-dose (875 mg/m2) levels. Investig New Drugs 15:115–121
Abbruzzese JL, Grunewald R, Weeks E, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber M (1991) A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 9:491–498
Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, Larvin M, Radstone D (2001) A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer. Health Technol Assess (Winch Eng) 5:1–70
Messaouda MB, Moussa F, Tangour B, Szwarc H, Abderrabba M (2007) Addition of bio-organic compounds on C 60: a semi-empirical investigation of its reactivity with glycine. J Mol Struct THEOCHEM 809:153–159
Haifa Khemir BT, Moussa F (2015) In silico study of spacer arm length influence on drug vectorization by fullerene C60. J Nanomater 2015:1–8
Mejri A, Vardanega D, Tangour B, Gharbi T, Picaud F (2014) Encapsulation into carbon nanotubes and release of anticancer cisplatin drug molecule. J Phys Chem B 119:604–611
Bessrour R, Belmiloud Y, Hosni Z, Tangour B (2012) Controlling drug efficiency by encapsulation into carbon nanotubes: a theoretical study of the antitumor cisplatin and the anti-HIV TIBO molecules. AIP Conf Proc 1456:229–239
Hosni Z, Bessrour R, Tangour B (2014) 195Pt chemical shift ability to control the antitumor drug cisplatin encapsulated into carbon nanotubes: a theoretical study. J Comput Theor Nanosci 11:318–323
Belmiloud Y, Ouraghi M, Brahimi M, Benaboura A, Charqaoui D, Tangour B (2012) Theoretical study of the anti-human Immuno-deficiency virus TIBO molecule confined into carbon nanotubes. J Comput Theor Nanosci 9:1101–1108
Stout DA (2015) Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr Pharm Des 21:2037–2044
Lay CL, Liu J, Liu Y (2011) Functionalized carbon nanotubes for anticancer drug delivery. Expert Rev Med Devices 8:561–566
Chechetka SA, Pichon B, Zhang M, Yudasaka M, Bégin-Colin S, Bianco A, Miyako E (2014) Multifunctional carbon nanohorn complexes for cancer treatment. Chem Asian J 10:160–165
Ajima K, Yudasaka M, Murakami T, Maigna A, Shiba K, Iijima S (2005) Carbon nanohorns as anticancer drug carriers. Mol Pharm 2:475–480
Khemir H, Tangour B, Moussa F (2016) An “in silico” study of drug vectorization by a carbon nanocone. J Comput Theor Nanosci 13:3384–3392
Duverger E, Gharbi T, Delabrousse E, Picaud F (2014) Quantum study of boron nitride nanotubes functionalized with anticancer molecules. Phys Chem Chem Phys 16:18425–18432
El Khalifi M, Duverger E, Gharbi T, Boulahdour H, Picaud F (2015) Theoretical demonstration of the potentiality of boron nitride nanotubes to encapsulate anticancer molecule. Phys Chem Chem Phys 17:30057–30064
Liu Z, Tabakman S, Welsher K, Dai H (2009) Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res 2:85–120
Kostarelos K, Bianco A, Prato M (2009) Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol 4:627–633
Jain KK (2007) Applications of nanobiotechnology in clinical diagnostics. Clin Chem 53:2002–2009
Prato M, Kostarelos K, Bianco A (2008) Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res 41:60–68
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171
Kim PS, Djazayeri S, Zeineldin R (2011) Novel nanotechnology approaches to diagnosis and therapy of ovarian cancer. Gynecol Oncol 120:393–403
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, Evmenenko G, Nguyen ST, Ruoff RS (2007) Preparation and characterization of graphene oxide paper. Nature 448:457–460
Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z (2010) Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett 10:3318–3323
Liu Z, Robinson JT, Sun X, Dai H (2008) PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc 130:10876–10877
Hondroulis E, Zhang Z, Chen C, Li C-Z (2012) Impedance based nanotoxicity assessment of graphene nanomaterials at the cellular and tissue level. Anal Lett 45:272–282
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9:9243–9257
Jiang T, Sun W, Zhu Q, Burns NA, Khan SA, Mo R, Gu Z (2014) Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv Mater 27:1021–1028
Zhou T, Zhou X, Xing D (2014) Controlled release of doxorubicin from graphene oxide-based charge-reversal nanocarrier. Biomaterials 35:4185–4194
Mo R, Jiang T, Sun W, Gu Z (2015) ATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug delivery. Biomaterials 50:67–74
Goenka S, Sant V, Sant S (2014) Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 173:75–88
Karlicky F, Kumara Ramanatha Datta K, Otyepka M, Zboril R (2013) Halogenated graphenes: rapidly growing family of graphene derivatives. ACS Nano 7:6434–6464
Yuk, J. M., Park, J., Ercius, P., Kim, K., Hellebusch, D. J., Crommie, M. F.,. Lee, J. Y, Zettl, A.,and Alivisatos, A. P. (2012) High-Resolution EM of Colloidal Nanocrystal Growth Using Graphene Liquid Cells. Science 336(6077): 61–64
Zhu S, Li T (2014) Hydrogenation-assisted graphene origami and its application in programmable molecular mass uptake, storage, and release. ACS Nano 8(3):2864–2872
Lu J, Neto AHC, Loh KP (2012) Transforming moiré blisters into geometric graphene nano-bubbles. Nat Commun 3:823
Levy N, Burke SA, Meaker KL, Panlasigui, Zettl A, Guinea F, Castro Neto AHC, Crommie MF (2010) Strain-induced pseudo–magnetic fields greater than 300 Tesla in graphene nanobubbles. Science 329:544–547
Bellido EP, Seminario JM (2010) Molecular dynamics simulations of folding of supported graphene. J Phys Chem C 114:22472–22477
Hill A, Sinner A, Ziegler K (2011) Valley symmetry breaking and gap tuning in graphene by spin doping. New J Phys 13:035023 (14)
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Ordejon P, Artacho E, Soler JM (1996) Self-consistent order-$N$ density-functional calculations for very large systems. Phys Rev B 53:R10441–R10444
Mallakpour S, Abdolmaleki A, Borandeh S (2014) Covalently functionalized graphene sheets with biocompatible natural amino acids. Appl Surf Sci 307:533–542
Muzi L, Ménard-Moyon C, Russier J, Li J, Chin CF, Ang WH, Pastorin G, Risuleo G, Bianco A (2015) Diameter-dependent release of a cisplatin pro-drug from small and large functionalized carbon nanotubes. Nano 7:5383–5394
Thomas M, Enciso M, Hilder T (2015) Insertion mechanism and stability of boron nitride nanotubes in lipid bilayers. J Phys Chem B 119(15):4929–4936
El Khalifi M, Bentin J, Duverger E, Gharbi T, Boulahdour H, Picaud F (2016) Encapsulation capacity and natural payload delivery of an anticancer drug from boron nitride nanotube. Phys Chem Chem Phys 18(36):24994–25001
Acknowledgements
Computations have been performed on the supercomputer facilities of the Mesocentre of the University of Franche-Comté. M. M. gratefully acknowledges the support provided by the Tunisian Ministry of High School and Scientific Research. The present work was financially supported by University of FrancheComté in its “accueil de jeunes chercheurs en séjour de recherche post-doctorale” program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mlaouah, M., Tangour, B., El Khalifi, M. et al. The encapsulation of the gemcitabine anticancer drug into grapheme nest: a theoretical study. J Mol Model 24, 102 (2018). https://doi.org/10.1007/s00894-018-3627-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3627-6