Abstract
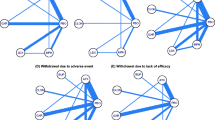
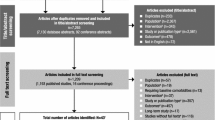
The aim of this study is to gather evidence of head-to-head double-blind randomized-controlled trials on the efficacy and safety of available treatments for attention deficit hyperactivity disorder (ADHD) in children and adolescents. A systematic review was conducted by two independent reviewers in ten electronic databases (PROSPERO register CRD42016043239). Methodological quality of included studies was evaluated according to the Jadad scale. Network meta-analyses were performed including double-blinded head-to-head trials comparing active allopathic drugs in patients (0–18 years old) diagnosed with ADHD. The results of efficacy and safety of atomoxetine (ATX), bupropion, buspirone (BSP), dexamphetamine, edivoxetine (EDX), guanfacine (GXR), lisdexamfetamine (LDX), methylphenidate (MPH), mixed amphetamine salts, modafinil, pindolol (PDL), reboxetine (RBX), selegiline, and venlafaxine were analyzed using ADDIS software v.1.16.5. Forty-eight trials were identified (n = 4169 participants), of which 12 were used for efficacy analysis and 33 for safety analysis. On the CGI-I scale, the analysis revealed that MPH was more effective than ATX and GXR. For the safety outcomes, according to drug ranks, LDX was more likely to cause sleep disorders (39%) as well as loss of appetite (65%) and behavior problems such as irritability (60%). BSP (71%) and EDX (44%) caused less appetite decrease. For behavioral effects, PDL was considered safest (50%). For any adverse events, RBX (89%) was the safest alternative. The lack of head-to-head trials properly reporting outcomes of interest limited some comparisons. Network meta-analysis offered a broader overview on the available treatments for ADHD, especially for safety issues, and contributes towards evidence gathering and clinical practice decisions. A core outcome set for ADHD should be designed to guide the conduction and report of clinical trials.





Similar content being viewed by others
References
Polanczyk GV et al (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43(2):434–442
Austerman J (2015) ADHD and behavioral disorders: assessment, management, and an update from DSM-5. Cleve Clin J Med 82(11 Suppl 1):S2–S7
Bruxel EM et al (2014) ADHD pharmacogenetics across the life cycle: new findings and perspectives. Am J Med Genet B Neuropsychiatr Genet 165B(4):263–282
Mohammadi MR, Mohammadzadeh S, Akhondzadeh S (2015) Memantine versus methylphenidate in children and adolescents with attention deficit hyperactivity disorder: a double-blind, randomized clinical trial. Iran J Psychiatry 10(2):106–114
Root RW II, Resnick RJ (2003) An update on the diagnosis and treatment of attention-deficit/hyperactivity disorder in children. Prof Psychol Res Pract 34(1):34
Lopresti AL (2015) Oxidative and nitrosative stress in ADHD: possible causes and the potential of antioxidant-targeted therapies. Atten Defic Hyperact Disord 7(4):237–247
Stevens JR, Wilens TE, Stern TA (2013) Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges. Prim Care Companion CNS Disord 15(2). https://doi.org/10.4088/PCC.12f01472
Stahl SM (2010) Mechanism of action of stimulants in attention-deficit/hyperactivity disorder. J Clin Psychiatry 71(1):12–13
Spiller HA, Hays HL, Aleguas A Jr (2013) Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs 27(7):531–543
Nakanishi Y et al (2017) Differential therapeutic effects of atomoxetine and methylphenidate in childhood attention deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Adolesc Psychiatry Ment Health 11:26
Storebo OJ, et al (2015) Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev (11):Cd009885
Rostain A et al (2015) Toward quality care in ADHD: defining the goals of treatment. J Atten Disord 19(2):99–117
Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ (2015) Clinical handbook of psychotropic drugs. Hogrefe Publishing Corporation/Hogrefe Publishing GmbH, Boston/Göttingen
Banaschewski T et al (2004) Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry 13(Suppl 1):I102–I116
Hennissen L et al (2017) Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs 31:1–17
Jafarinia M et al (2012) Bupropion versus methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder: randomized double-blind study. Hum Psychopharmacol 27(4):411–418
Akhondzadeh S et al (2003) Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. Prog Neuropsychopharmacol Biol Psychiatry 27(5):841–845
Arabgol F, Panaghi L, Hebrani P (2009) Reboxetine versus methylphenidate in treatment of children and adolescents with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry 18(1):53–59
Mohammadi MR et al (2010) Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double-blind trial. Hum Psychopharmacol 25(7–8):560–565
Zarinara AR et al (2010) Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol 25(7–8):530–535
Song F et al (2009) Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 338:b1147
Song F et al (2011) Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ 343:d4909
Tonin FS et al (2015) Adverse events and treatment failure leading to discontinuation of recently approved antipsychotic drugs in schizophrenia: a network meta-analysis. Schizophr Res 169(1):483–485
Tonin FS et al (2017) Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract 1(1):943
Cortese S et al (2017) Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open 7(1):e013967
Punja S et al (2016) To meta-analyze or not to meta-analyze? A combined meta-analysis of N-of-1 trial data with RCT data on amphetamines and methylphenidate for pediatric ADHD. J Clin Epidemiol 76:76–81
Camporeale A et al (2015) Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol 29(1):3–14
Chan E, Fogler JM, Hammerness PG (2016) Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA J Am Med Assoc 315(18):1997–2008
Cohen SC et al (2015) Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry 54(9):728–736
Maneeton B et al (2015) Comparative efficacy, acceptability, and tolerability of lisdexamfetamine in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Drug Des Dev Ther 9:1927–1936
Maneeton N et al (2015) A systematic review of dexmethylphenidate versus placebo in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Eur Neuropsychopharmacol 25:S642
Joseph A et al (2017) Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry 26:1–23
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane
Hutton B et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Jadad AR et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Jansen JP et al (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14(4):417–428
Dias S et al (2013) Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Mak 33(5):641–656
Weiss G et al (1971) Comparison of the effects of chlorpromazine, dextroamphetamine and methylphenidate on the behaviour and intellectual functioning of hyperactive children. Can Med Assoc J 104(1):20–25
Arnold LE et al (1972) Levoamphetamine and dextroamphetamine: comparative efficacy in the hyperkinetic syndrome. Assessment by target symptoms. Arch Gen Psychiatry 27(6):816–822
Huestis RD, Arnold LE, Smeltzer DJ (1975) Caffeine versus methylphenidate and d-amphetamine in minimal brain dysfunction: a double-blind comparison. Am J Psychiatry 132(8):868–870
Garfinkel BD, Webster CD, Sloman L (1975) Methylphenidate and caffeine in the treatment of children with minimal brain dysfunction. Am J Psychiatry 132(7):723–728
Arnold LE et al (1978) Methylphenidate vs dextroamphetamine vs caffeine in minimal brain dysfunction: controlled comparison by placebo washout design with Bayes’ analysis. Arch Gen Psychiatry 35(4):463–473
Arnold LE et al (1976) Levoamphetamine vs dextroamphetamine in minimal brain dysfunction. Replication, time response, and differential effect by diagnostic group and family rating. Arch Gen Psychiatry 33(3):292–301
Conners CK, Taylor E (1980) Pemoline, methylphenidate, and placebo in children with minimal brain dysfunction. Arch Gen Psychiatry 37(8):922–930
Butter HJ et al (1983) A comparative study of the efficacy of ACTH4-9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. J Clin Psychopharmacol 3(4):226–230
Garfinkel BD et al (1983) Tricyclic antidepressant and methylphenidate treatment of attention deficit disorder in children. J Am Acad Child Psychiatry 22(4):343–348
Donnelly M et al (1989) Fenfluramine and dextroamphetamine treatment of childhood hyperactivity. Clinical and biochemical findings. Arch Gen Psychiatry 46(3):205–212
Zametkin A et al (1985) Treatment of hyperactive children with monoamine oxidase inhibitors. I. Clinical efficacy. Arch Gen Psychiatry 42(10):962–966
Pelham WE Jr et al (1990) Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: a comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, and pemoline. Pediatrics 86(2):226–237
Elia J et al (1993) Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. J Child Psychol Psychiatry 34(5):785–804
Barrickman LL et al (1995) Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34(5):649–657
Buitelaar JK et al (1996) Pindolol and methylphenidate in children with attention-deficit hyperactivity disorder. Clinical efficacy and side-effects. J Child Psychol Psychiatry 37(5):587–595
Efron D, Jarman F, Barker M (1997) Methylphenidate versus dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 100(6):E6
Pelham WE et al (1999) A comparison of ritalin and adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics 103(4):e43
Pelham WE et al (1999) A comparison of morning-only and morning/late afternoon adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 104(6):1300–1311
Pliszka SR et al (2000) A double-blind, placebo-controlled study of adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 39(5):619–626
James RS et al (2001) Double-blind, placebo-controlled study of single-dose amphetamine formulations in ADHD. J Am Acad Child Adolesc Psychiatry 40(11):1268–1276
Overtoom CC et al (2003) Effects of methylphenidate, desipramine, and l-dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behav Brain Res 145(1–2):7–15
Mohammadi MR et al (2004) Selegiline in comparison with methylphenidate in attention deficit hyperactivity disorder children and adolescents in a double-blind, randomized clinical trial. J Child Adolesc Psychopharmacol 14(3):418–425
Mohammadi MR et al (2004) Efficacy of theophylline compared to methylphenidate for the treatment of attention-deficit hyperactivity disorder in children and adolescents: a pilot double-blind randomized trial. J Clin Pharm Ther 29(2):139–144
Wigal S et al (2004) A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43(11):1406–1414
Wigal SB et al (2005) A laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord 9(1):275–289
Sangal RB et al (2006) Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep 29(12):1573–1585
Biederman J et al (2007) Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry 62(9):970–976
Wang Y et al (2007) Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry 41(3):222–230
Amiri S et al (2008) Modafinil as a treatment for attention-deficit/hyperactivity disorder in children and adolescents: a double blind, randomized clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 32(1):145–149
Newcorn JH et al (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165(6):721–730
Daviss WB et al (2008) Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry 47(2):189–198
Palumbo DR et al (2008) Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry 47(2):180–188
Nair V, Mahadevan S (2009) Randomised controlled study-efficacy of clonidine versus carbamazepine in children with ADHD. J Trop Pediatr 55(2):116–121
Davari-Ashtiani R et al (2010) Buspirone versus methylphenidate in the treatment of attention deficit hyperactivity disorder: a double-blind and randomized trial. Child Psychiatry Hum Dev 41(6):641–648
Stein MA et al (2011) Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol 21(6):581–588
Mohammadi MR et al (2012) Buspirone versus methylphenidate in the treatment of children with attention- deficit/hyperactivity disorder: randomized double-blind study. Acta Med Iran 50(11):723–728
Coghill D et al (2013) European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 23(10):1208–1218
Santisteban JA et al (2014) Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs 28(9):825–833
Lin DY et al (2014) A randomized trial of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24(4):190–200
Dittmann RW et al (2013) Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs 27(12):1081–1092
Nagy P et al (2016) Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder and an inadequate response to methylphenidate. Eur Child Adolesc Psychiatry 25(2):141–149
Arabgol F, Panaghi L, Nikzad V (2015) Risperidone versus methylphenidate in treatment of preschool children with attention-deficit hyperactivity disorder. Iran J Pediatr 25(1):e265
McCracken JT et al (2016) Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry 55(8):657.e1–666.e1
Huss M et al (2015) Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, double-blind, multicentre, placebo- and active-reference phase 3 study. Aust N Z J Psychiatry 49:111
Efron D, Jarman F, Barker M (1997) Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 100(4):662–666
ATTENTION-DEFICIT SO (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011–2654
Rezaei G et al (2016) Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review and meta-analysis. Med J Islam Repub Iran 30:325
Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. Lancet 387(10024):1240–1250
Chierrito de Oliveira D, et al (2017) Safety of treatments for ADHD in adults: pairwise and network meta-analyses. J Atten Disord. https://doi.org/10.1177/1087054717696773
Apter JT, Allen LA (1999) Buspirone: future directions. J Clin Psychopharmacol 19(1):86–93
Coghill DR et al (2014) Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry 23(2):61–68
Cohen-Yavin I et al (2009) Efficacy of reboxetine in the treatment of attention-deficit/hyperactivity disorder in boys with intolerance to methylphenidate: an open-label, 8-week, methylphenidate-controlled trial. Clin Neuropharmacol 32(4):179–182
Page ME (2003) The promises and pitfalls of reboxetine. CNS Drug Rev 9(4):327–342
Eyding D et al (2010) Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 341:c4737
Preskorn SH (2004) Reboxetine: a norepinephrine selective reuptake pump inhibitor. J Psychiatr Pract 10(1):57–63
Sepede G et al (2012) Reboxetine in clinical practice: a review. Clin Ter 163(4):e255–e262
Ghanizadeh A (2015) A systematic review of reboxetine for treating patients with attention deficit hyperactivity disorder. Nord J Psychiatry 69(4):241–248
Kirkham JJ et al (2013) Can a core outcome set improve the quality of systematic reviews?–a survey of the Co-ordinating Editors of Cochrane Review Groups. Trials 14:21
Author information
Authors and Affiliations
Contributions
SV and RP conceived and designed the study; SCOSP, FST, and HHLB acquired and analyzed data; SCOSP, FST, and HHLB drafted the manuscript; SV and RP read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padilha, S.C.O.S., Virtuoso, S., Tonin, F.S. et al. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry 27, 1335–1345 (2018). https://doi.org/10.1007/s00787-018-1125-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-018-1125-0