Abstract
Objectives
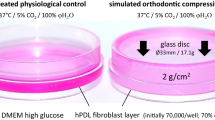
The aim was to investigate the impact of static compressive force (CF) application on human PDL-derived fibroblasts (HPDF) in vitro for up to 6 days on the expression of specific genes and to monitor cell growth and cell viability.
Materials and methods
CF of 2 g/cm2 was applied on HPDFs for 1–6 days. On each day, gene expression (cFOS, HB-GAM, COX2, IL6, TNFα, RUNX2, and P2RX2) and secretion (TNFα, PGE2) were determined by RT-qPCR and ELISA, respectively. Cell growth and cell viability were monitored daily.
Results
In comparison with controls, significant upregulation of cFOS in compressed HPDFs was observed. HB-GAM showed no changes in expression, except on day 5 (P < 0.001). IL6 expression was significantly upregulated from day 2–5, reaching the maximum on day 3 (P < 0.001). TNFα expression was upregulated on all but day 2. COX2 showed upregulation, reaching the plateau from day 3 (P < 0.001) until day 4 (P < 0.001), and returning to the initial state till day 6. P2RX7 was downregulated on days 2 and 4 to 6 (P < 0.001). RUNX2 was downregulated on days 2 and 5 (both P < 0.001). Cells in both groups were proliferating, and no negative effect on cell viability was observed.
Conclusion
Results suggest high molecular activity up to 6 days, therefore introducing further need for in vitro studies with a longer duration that would explain other genes and metabolites involved in orthodontic tooth movement (OTM).
Clinical relevance
Extension of an established in vitro force application system for prolonged force application (6 days) simulating the initial phase of OTM.






Similar content being viewed by others
References
Reitan K (1960) Tissue behavior during orthodontic tooth movement. Am J Orthod 46(12):881–900. https://doi.org/10.1016/0002-9416(60)90091-9
Davidovitch Z (1991) Tooth movement. Crit Rev Oral Biol Med 2(4):411–450. https://doi.org/10.1177/10454411910020040101
Marchesan JT, Scanlon CS, Soehren S, Matsuo M, Kapila YL (2011) Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch Oral Biol 56(10):933–943. https://doi.org/10.1016/j.archoralbio.2011.03.003
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop 129(4):469.e1–469.32. https://doi.org/10.1016/j.ajodo.2005.10.007
Yang L, Yang Y, Wang S, Li Y, Zhao Z (2015) In vitro mechanical loading models for periodontal ligament cells: from two-dimensional to three-dimensional models. Arch Oral Biol 60(3):416–424. https://doi.org/10.1016/j.archoralbio.2014.11.012
Kanai K, Nohara H, Hanada K (1992) Initial effects of continuously applied compressive stress to human periodontal ligament fibroblasts. Nihon Kyosei Shika Gakkai Zasshi 51:153–163
Kanzaki H, Chiba M, Shimizu Y, Mitani H (2002) Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor κB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 17(2):210–220. https://doi.org/10.1359/jbmr.2002.17.2.210
Nakajima R, Yamaguchi M, Kojima T, Takano M, Kasai K (2008) Effects of compression force on fibroblast growth factor-2 and receptor activator of nuclear factor kappa B ligand production by periodontal ligament cells in vitro. J Periodontal Res 43(2):168–173. https://doi.org/10.1111/j.1600-0765.2007.01008.x
Janjic M, Docheva D, Trickovic Janjic O, Wichelhaus A, Baumert U (2018) In vitro weight-loaded cell models for understanding mechanodependent molecular pathways involved in orthodontic tooth movement: a systematic review. Stem Cells Int 2018:3208285. https://doi.org/10.1155/2018/3208285
Vansant L, Cadenas De Llano-Perula M, Verdonck A, Willems G (2018) Expression of biological mediators during orthodontic tooth movement: a systematic review. Arch Oral Biol 95:170–186. https://doi.org/10.1016/j.archoralbio.2018.08.003
Schröder A, Bauer K, Spanier G, Proff P, Wolf M, Kirschneck C (2018) Expression kinetics of human periodontal ligament fibroblasts in the early phases of orthodontic tooth movement. J Orofac Orthop 79(5):337–351. https://doi.org/10.1007/s00056-018-0145-1
Somerman MJ, Archer SY, Imm GR, Foster RA (1988) A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J Dent Res 67(1):66–70. https://doi.org/10.1177/00220345880670011301
Ng KW, Schantz J-T (2010) A manual for primary human cell culture, vol 6. Manuals in Biomedical Research, 2nd edn. World Scientific, Hackensack
Docheva D, Padula D, Popov C, Weishaupt P, Prägert M, Miosge N, Hickel R, Böcker W, Clausen-Schaumann H, Schieker M (2010) Establishment of immortalized periodontal ligament progenitor cell line and its behavioural analysis on smooth and rough titanium surface. Eur Cell Mater 19:228–241. https://doi.org/10.22203/eCM.v019a22
Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S (2010) MIQE précis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 11:74. https://doi.org/10.1186/1471-2199-11-74
Shi J, Baumert U, Folwaczny M, Wichelhaus A (2019) Influence of static forces on the expression of selected parameters of inflammation in periodontal ligament cells and alveolar bone cells in a co-culture in vitro model. Clin Oral Investig 23(6):2617–2628. https://doi.org/10.1007/s00784-018-2697-2
Friedl G, Schmidt H, Rehak I, Kostner G, Schauenstein K, Windhager R (2007) Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: transcriptionally controlled early osteo-chondrogenic response in vitro. Osteoarthr Cartil 15(11):1293–1300. https://doi.org/10.1016/j.joca.2007.04.002
Bustin SA (2012) Definitive qPCR: assay design. Version: v.1.6 (02 march 2013). URL: https://sellfy.com/p/iQfl/ (URL accessed 11-06-2019)
Thornton B, Basu C (2015) Rapid and simple method of qPCR primer design. Methods Mol Biol 1275:173–179. https://doi.org/10.1007/978-1-4939-2365-6_13
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Hellemans J, Vandesompele J (2011) Quantitative PCR data analysis - unlocking the secret to successful results. In: Kennedy S, Oswald N (eds) PCR troubleshooting and optimization : the essential guide. Caister Academic Press, Norfolk, pp 139–150
Kang KL, Lee SW, Ahn YS, Kim SH, Kang YG (2013) Bioinformatic analysis of responsive genes in two-dimension and three-dimension cultured human periodontal ligament cells subjected to compressive stress. J Periodontal Res 48(1):87–97. https://doi.org/10.1111/j.1600-0765.2012.01507.x
Benjakul S, Jitpukdeebodintra S, Leethanakul C (2018) Effects of low magnitude high frequency mechanical vibration combined with compressive force on human periodontal ligament cells in vitro. Eur J Orthod 40(4):356–363. https://doi.org/10.1093/ejo/cjx062
Bougault C, Aubert-Foucher E, Paumier A, Perrier-Groult E, Huot L, Hot D, Duterque-Coquillaud M, Mallein-Gerin F (2012) Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS One 7(5):e36964. https://doi.org/10.1371/journal.pone.0036964
Bao X, Clark CB, Frangos JA (2000) Temporal gradient in shear-induced signaling pathway: involvement of MAP kinase, c-fos, and connexin43. Am J Physiol Heart Circ Physiol 278(5):H1598–H1605. https://doi.org/10.1152/ajpheart.2000.278.5.H1598
Kletsas D, Basdra EK, Papavassiliou AG (2002) Effect of protein kinase inhibitors on the stretch-elicited c-Fos and c-Jun up-regulation in human PDL osteoblast-like cells. J Cell Physiol 190(3):313–321. https://doi.org/10.1002/jcp.10052
Papadopoulou A, Iliadi A, Eliades T, Kletsas D (2017) Early responses of human periodontal ligament fibroblasts to cyclic and static mechanical stretching. Eur J Orthod 39(3):258–263. https://doi.org/10.1093/ejo/cjw075
Yamaguchi N, Chiba M, Mitani H (2002) The induction of c-fos mRNA expression by mechanical stress in human periodontal ligament cells. Arch Oral Biol 47(6):465–471. https://doi.org/10.1016/S0003-9969(02)00022-5
Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D (2015) Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol 16:6. https://doi.org/10.1186/s12867-015-0036-6
Imai S, Heino TJ, Hienola A, Kurata K, Buki K, Matsusue Y, Vaananen HK, Rauvala H (2009) Osteocyte-derived HB-GAM (pleiotrophin) is associated with bone formation and mechanical loading. Bone 44(5):785–794. https://doi.org/10.1016/j.bone.2009.01.004
Imai S, Kaksonen M, Raulo E, Kinnunen T, Fages C, Meng X, Lakso M, Rauvala H (1998) Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM). J Cell Biol 143(4):1113–1128. https://doi.org/10.1083/jcb.143.4.1113
Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S (2005) Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96(5):1049–1060. https://doi.org/10.1002/jcb.20606
Grant M, Wilson J, Rock P, Chapple I (2013) Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic tooth movement. Eur J Orthod 35(5):644–651. https://doi.org/10.1093/ejo/cjs057
Kunii R, Yamaguchi M, Tanimoto Y, Asano M, Yamada K, Goseki T, Kasai K (2013) Role of interleukin-6 in orthodontically induced inflammatory root resorption in humans. Korean J Orthod 43(6):294–301. https://doi.org/10.4041/kjod.2013.43.6.294
Yamada K, Yamaguchi M, Asano M, Fujita S, Kobayashi R, Kasai K (2013) Th17-cells in atopic dermatitis stimulate orthodontic root resorption. Oral Dis 19(7):683–693. https://doi.org/10.1111/odi.12053
Kirschneck C, Proff P, Maurer M, Reicheneder C, Römer P (2015) Orthodontic forces add to nicotine-induced loss of periodontal bone : an in vivo and in vitro study. J Orofac Orthop 76(3):195–212. https://doi.org/10.1007/s00056-015-0283-7
Proff P, Reicheneder C, Faltermeier A, Kubein-Meesenburg D, Römer P (2014) Effects of mechanical and bacterial stressors on cytokine and growth-factor expression in periodontal ligament cells. J Orofac Orthop 75(3):191–202. https://doi.org/10.1007/s00056-014-0212-1
Kim SJ, Park KH, Park YG, Lee SW, Kang YG (2013) Compressive stress induced the up-regulation of M-CSF, RANKL, TNF-a expression and the down-regulation of OPG expression in PDL cells via the integrin-FAK pathway. Arch Oral Biol 58(6):707–716. https://doi.org/10.1016/j.archoralbio.2012.11.003
Mitsuhashi M, Yamaguchi M, Kojima T, Nakajima R, Kasai K (2011) Effects of HSP70 on the compression force-induced TNF-a and RANKL expression in human periodontal ligament cells. Inflamm Res 60(2):187–194. https://doi.org/10.1007/s00011-010-0253-x
Brändström H, Jonsson KB, Ohlsson C, Vidal O, Ljunghall S, Ljunggren Ö (1998) Regulation of osteoprotegerin mRNA levels by prostaglandin E2 in human bone marrow stroma cells. Biochem Biophys Res Commun 247(2):338–341. https://doi.org/10.1006/bbrc.1998.8783
Kang YG, Nam JH, Kim KH, Lee KS (2010) FAK pathway regulates PGE2 production in compressed periodontal ligament cells. J Dent Res 89(12):1444–1449. https://doi.org/10.1177/0022034510378521
Mayahara K, Kobayashi Y, Takimoto K, Suzuki N, Mitsui N, Shimizu N (2007) Aging stimulates cyclooxygenase-2 expression and prostaglandin E2 production in human periodontal ligament cells after the application of compressive force. J Periodontal Res 42(1):8–14. https://doi.org/10.1111/j.1600-0765.2006.00885.x
Kirschneck C, Meier M, Bauer K, Proff P, Fanghänel J (2017) Meloxicam medication reduces orthodontically induced dental root resorption and tooth movement velocity: a combined in vivo and in vitro study of dental-periodontal cells and tissue. Cell Tissue Res 368(1):61–78. https://doi.org/10.1007/s00441-016-2553-0
Mayahara K, Yamaguchi A, Sakaguchi M, Igarashi Y, Shimizu N (2010) Effect of Ga-Al-As laser irradiation on COX-2 and cPLA2-a expression in compressed human periodontal ligament cells. Lasers Surg Med 42(6):489–493. https://doi.org/10.1002/lsm.20871
Grimm S, Wolff E, Walter C, Pabst AM, Mundethu A, Jacobs C, Wehrbein H, Jacobs C (2019) Influence of clodronate and compressive force on IL-1ss-stimulated human periodontal ligament fibroblasts. Clin Oral Investig:1–8. https://doi.org/10.1007/s00784-019-02930-z
Wada S, Kanzaki H, Narimiya T, Nakamura Y (2017) Novel device for application of continuous mechanical tensile strain to mammalian cells. Biol Open 6(4):518–524. https://doi.org/10.1242/bio.023671
Kariya T, Tanabe N, Shionome C, Manaka S, Kawato T, Zhao N, Maeno M, Suzuki N, Shimizu N (2015) Tension force-induced ATP promotes osteogenesis through P2X7 receptor in osteoblasts. J Cell Biochem 116(1):12–21. https://doi.org/10.1002/jcb.24863
Shen T, Qiu L, Chang H, Yang Y, Jian C, Xiong J, Zhou J, Dong S (2014) Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int J Clin Exp Pathol 7(11):7872–7880
Wei F, Liu D, Feng C, Zhang F, Yang S, Hu Y, Ding G, Wang S (2015) microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev 24(3):312–319. https://doi.org/10.1089/scd.2014.0191
Lee SY, Yoo HI, Kim SH (2015) CCR5-CCL axis in PDL during orthodontic biophysical force application. J Dent Res 94(12):1715–1723. https://doi.org/10.1177/0022034515603926
Viecilli RF, Katona TR, Chen J, Hartsfield JK Jr, Roberts WE (2009) Orthodontic mechanotransduction and the role of the P2X7 receptor. Am J Orthod Dentofacial Orthop 135(6):694.e1–694.16; discussion 694-695. https://doi.org/10.1016/j.ajodo.2008.10.018
Kanjanamekanant K, Luckprom P, Pavasant P (2013) Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J Periodontal Res 48(2):169–176. https://doi.org/10.1111/j.1600-0765.2012.01517.x
Xu XY, He XT, Wang J, Li X, Xia Y, Tan YZ, Chen FM (2019) Role of the P2X7 receptor in inflammation-mediated changes in the osteogenesis of periodontal ligament stem cells. Cell Death Dis 10(1):20. https://doi.org/10.1038/s41419-018-1253-y
Jacobs C, Walter C, Ziebart T, Grimm S, Meila D, Krieger E, Wehrbein H (2014) Induction of IL-6 and MMP-8 in human periodontal fibroblasts by static tensile strain. Clin Oral Investig 18(3):901–908. https://doi.org/10.1007/s00784-013-1032-1
Nazet U, Schröder A, Spanier G, Wolf M, Proff P, Kirschneck C (2019) Simplified method for applying static isotropic tensile strain in cell culture experiments with identification of valid RT-qPCR reference genes for PDL fibroblasts. Eur J Orthod. https://doi.org/10.1093/ejo/cjz052
Nettelhoff L, Grimm S, Jacobs C, Walter C, Pabst AM, Goldschmitt J, Wehrbein H (2016) Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin Oral Investig 20(3):621–629. https://doi.org/10.1007/s00784-015-1542-0
Shi J, Folwaczny M, Wichelhaus A, Baumert U (2019) Differences in RUNX2 and P2RX7 gene expression between mono- and coculture of human periodontal ligament cells and human osteoblasts under compressive force application. Orthod Craniofacial Res 23(6):2617–2628. https://doi.org/10.1111/ocr.12307
Yamaguchi M, Garlet GP (2015) The role of inflammation in defining the type and pattern of tissue response in orthodontic tooth movement. In: Krishnan V, Davidovitch Z (eds) Biological mechanisms of tooth movement, 2nd edn. Wiley Blackwell, Chichester, pp 121–137
Baumert U, Golan I, Becker B, Hrala BP, Redlich M, Roos HA, Palmon A, Reichenberg E, Müßig D (2004) Pressure simulation of orthodontic force in osteoblasts: a pilot study. Orthod Craniofacial Res 7(1):3–9. https://doi.org/10.1046/j.1601-6335.2003.00270.x
Yamaguchi M, Shimizu N, Ozawa Y, Saito K, Miura S, Takiguchi H, Iwasawa T, Abiko Y (1997) Effect of tension-force on plasminogen activator activity from human periodontal ligament cells. J Periodontal Res 32(3):308–314. https://doi.org/10.1111/j.1600-0765.1997.tb00539.x
Kirschneck C, Proff P, Fanghänel J, Wolf M, Roldan JC, Römer P (2016) Reference genes for valid gene expression studies on rat dental, periodontal and alveolar bone tissue by means of RT-qPCR with a focus on orthodontic tooth movement and periodontitis. Ann Anat 204:93–105. https://doi.org/10.1016/j.aanat.2015.11.005
Kirschneck C, Batschkus S, Proff P, Köstler J, Spanier G, Schröder A (2017) Valid gene expression normalization by RT-qPCR in studies on hPDL fibroblasts with focus on orthodontic tooth movement and periodontitis. Sci Rep 7(1):14751. https://doi.org/10.1038/s41598-017-15281-0
Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, Rajfer J, Gonzalez-Cadavid NF (2005) Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for Peyronie disease. Biol Reprod 73(6):1199–1210. https://doi.org/10.1095/biolreprod.105.041038
Acknowledgments
The authors wish to thank Christine Schreindorfer and Lisa Müller (both from the Dept. of Orthodontics, LMU Munich) for their assistance in the lab work.
Funding
M.J.R. was funded by BAYHOST (Bayerisches Hochschulzentrum für Mittel-, Ost- und Südosteuropa, Regensburg, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Janjic Rankovic, M., Docheva, D., Wichelhaus, A. et al. Effect of static compressive force on in vitro cultured PDL fibroblasts: monitoring of viability and gene expression over 6 days. Clin Oral Invest 24, 2497–2511 (2020). https://doi.org/10.1007/s00784-019-03113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03113-6