Abstract
Objectives
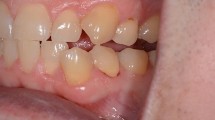
To assess the clinical effectiveness of four desensitizing materials in patients who are xerostomic due to radiotherapy for head and neck cancer (HNC) in comparison to a healthy group with normal salivation.
Methods and materials
The study was conducted as a split-mouth randomized clinical trial. Forty HNC patients (group A) and 46 healthy patients (group B) suffering from dentin hypersensitivity (DH) were included. Salivary flow was determined through a scialometric test. Hypersensitivity was assessed with air stimulus and tactile stimulus. The materials used as desensitizing agents were Vertise Flow, Universal Dentin Sealant, Clearfil Protect Bond, and Flor-Opal Varnish. The response was recorded before application of the materials, immediately after, and at 1 week, 4 weeks, and 12 weeks.
Results
Salivary flow rates in groups A/B were 0.15/0.53 mL/min (unstimulated) and 0.54/1.27 mL/min (stimulated), respectively. In group A, 100 hypersensitive teeth were included. Application of the desensitizing agents significantly decreased the hypersensitivity immediately and throughout the 4-week follow-up (p < 0.001). However, after the 12-week timepoint, a loss of efficacy was detected in all agents (p = 0.131). In group B, 116 hypersensitive teeth were included. The materials performed a more stable action, although a loss of effectiveness was detected at 12-week control (p = 0.297).
Conclusion
The efficiency of the desensitizing agents after the first application was similar in both groups. In the radiated group, this effect lasted for shorter periods than in healthy controls.
Clinical relevance
HNC patients with hyposalivation may be a new risk group for DH.



Similar content being viewed by others
References
Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R (2011) Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev 7:CD008934. https://doi.org/10.1002/14651858.CD008934.pub2
Pinna R, Campus G, Cumbo E, Mura I, Milia E (2015) Xerostomia induced by radiotherapy: an overview of the physio-pathology, clinical evidence and management of the oral damage. The Clin Risk Manag 4:171–188. https://doi.org/10.2147/TCRM.S70652
López-Jornet MP, García-Teresa G, Viñas M, Vinuesa T (2011) Clinical and antimicrobial evaluation of a mouthwash and toothpaste for xerostomia: a randomized, double-blind, crossover study. J Dent 39:757–763. https://doi.org/10.1016/j.jdent.2011.08.007
Hahnel S, Schwarz S, Zeman F, Schäfer L, Behr M (2014) Prevalence of xerostomia and hyposalivation and their association with quality of life in elderly patients in dependence on dental status and prosthetic rehabilitation: a pilot study. J Dent 42:664–670. https://doi.org/10.1016/j.jdent.2014.03.003
Chambers MS, Rosenthal DI, Weber RS (2007) Radiation-induced xerostomia. Head Neck 29:58–63
Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P (2001) Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 23:389–398
Miller EH, Quinn AI (2006) Dental considerations in the management of head and neck cancer patients. Otolaryngol Clin N Am 39:319–329
Ben-Aryeh H, Miron D, Berdicevsky I, Szargel R, Gutman D (1985) Xerostomia in the elderly: prevalence, diagnosis, complications and treatment. Gerodontology 4:77–82
Almståhl A, Wikström M (1998) Oral microflora in subjects with reduced salivary secretion. J Dent Res 78:1410–1416
Lingström P, Birkhed D (1993) Plaque pH and oral retention after consumption of starchy snack products at normal and low salivary secretion rate. Acta Odontol Scand 51:379–438
Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G (2013) The use of probiotic strains in caries prevention: a systematic review. Nutrients 5:2530–2550. https://doi.org/10.3390/nu5072530
Delaviz Y, Finer Y, Santerre JP (2014) Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater 30:16–32. https://doi.org/10.1016/j.dental.2013.08.201
Milia E, Pinna R, Filigheddu E, Eramo S (2016) Adhesive restorations and the oral environmental behaviour. In: Rudawska A (ed) Adhesives–applications and properties. Rijeka, InTech, pp 137–165
Brännström M (1966) Sensitivity of dentine. Oral Surg Oral Med Oral Pathol 21:517–526
Jones SB, Parkinson CR, Jeffery P, Davies M, Macdonald EL, Seong J et al (2015) A randomised clinical trial investigating calcium sodium phosphosilicate as a dentine mineralising agent in the oral environment. J Dent 43:757–764. https://doi.org/10.1016/j.jdent.2014.10.005
Brännström M, Linden LA, Johnson G (1968) Movement of dentinal and pulpal fluid caused by clinical procedures. J Dent Res 47:679–682
Van Loveren C (2013) Exposed cervical dentin and dentin hypersensitivity summary of the discussion and recommendations. Clin Oral Investig 17:S73–S76. https://doi.org/10.1007/s00784-012-0902-2
Mantzourani M, Sharma D (2013) Dentine sensitivity: past, present and future. J Dent 41:S3–S17. https://doi.org/10.1016/S0300-5712(13)70002-2
Pashley DH (1986) Dentine permeability, dentine sensitivity and treatment through tubule occlusion. J End 12:465–474
Pamir T, Özyazici M, Baloglu E, Önal B (2005) The efficacy of three desensitizing agents in treatment of dentine hypersensitivity. J Clin Pharm Ther 30:73–76
Duran I, Sengun A (2004) The long-term effectiveness of five current desensitizing products on cervical dentine sensitivity. J Oral Rehabil 31:351–356
Vano M, Derchi G, Barone A, Pinna R, Usai P, Covani (2018) Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: a double-blind randomized controlled trial. Clin Oral Investig 22:313–320. https://doi.org/10.1007/s00784-017-2113-3
Milia E, Castelli G, Bortone A, Sotgiu G, Manunta A, Pinna R, Gallina G (2012) Short-term response of three resin-based materials as desensitizing agents under oral environmental exposure. Acta Odontol Scand 71:599–609. https://doi.org/10.3109/00016357.2012.700063
Pinna R, Bortone A, Sotgiu G, Dore S, Usai P, Milia E (2015) Clinical evaluation of the efficacy of one self-adhesive composite in dental hypersensitivity. Clin Oral Investig 19:1663–1672. https://doi.org/10.1007/s00784-014-1390-3
Sreebny LM (1996) Xerostomia: diagnosis, management and clinical complications. In: Edgar WM, O’Mullane DM (eds) Saliva and oral health, 2nd edn. British Dental Association, London, pp 43–66
Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M (2007) Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22:21–24
Ritter AV, de L Dias W, Miguez P, Caplan DJ, Swift EJ Jr (2006) Treating cervical dentin hypersensitivity with fluoride varnish. A randomized clinical study. J Am Dent Assoc 137:1013–1020
Wang L, Magalhães AC, Francisconi-Dos-Rios LF, Calabria MP, Araújo D, Buzalaf M, Lauris J, Pereira JC (2016) Treatment of dentin hypersensitivity using Nano-hydroxyapatite pastes: a randomized three-month clinical trial. Oper Dent 41:E93–E101. https://doi.org/10.2341/15-145-C
Splieth CH, Tachou A (2013) Epidemiology of dentin hypersensitivity. Clin Oral Investig 17:S3–S8. https://doi.org/10.1007/s00784-012-0889-8
Loh SY, Mcleod RW, Elhassan HA (2017) Trismus following different treatment modalities for head and neck cancer: a systematic review of subjective measures. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-017-4519-6
Rees JS, Addy M (2004) A cross-sectional study of buccal cervical sensitivity in UK general dental practice and a summary review of prevalence studies. Int J Dent Hyg 2:64–69
McComb D, Erickson RL, Maxymiw WG, Wood RE (2002) A clinical comparison of glass ionomer, resin-modified glass ionomer and resin composite restorations in the treatment of cervical caries in xerostomic head and neck radiation patients. Oper Dent 27:430–437
Alghilan MA, Cook NB, Platt JA, Eckert GJ, Hara AT (2015) Susceptibility of restorations and adjacent enamel/dentine to erosion under different salivary flow conditions. J Dent 43:1476–1482. https://doi.org/10.1016/j.jdent.2015.10.007
Kielbassa AM, Muntz I, Bruggmoser G, Schulte-Monting J (2002) Effect of demineralization and remineralization on microhardness of irradiated dentin. J Clin Dent 13:104–110
Fränzel W, Gerlach R, Hein HJ, Schaller HG (2006) Effect of tumour therapeutic irradiation on the mechanical properties of teeth tissue. Z Med Phys 16:148–154
Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI (2002) Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck 24:737–747
Keilbassa AM et al; Grötz KA, Duschner H, Kutzner J, Thelen M, Wagner W (1998) Histographic study of the direct effects of radiation on dental enamel. Mund Kiefer Gesichts Chir 2:85–90
Galetti R, Santos-Silva AR, Antunes AN, Alves Fde A, Lopes MA, de Goes MF (2014) Radiotherapy does not impair dentin adhesive properties in head and neck cancer patients. Clin Oral Investig 18:1771–1778. https://doi.org/10.1007/s00784-013-1155-4
da Cunha SR, Ramos PA, Haddad CM, da Silva JL, Fregnani ER, Aranha AC (2016) Effects of different radiation doses on the bond strengths of two different adhesive systems to enamel and dentin. J Adhes Dent 18(2):151–156. https://doi.org/10.3290/j.jad.a35841
Lieshout HF, Bots CP (2014) The effect of radiotherapy on dental hard tissue--a systematic review. Clin Oral Investig 18:17–24. https://doi.org/10.1007/s00784-013-1034-z
Bernard C, Villat C, Abouelleil H, Gustin MP, Grosgogeat B (2015) Tensile bond strengths of two adhesives on irradiated and nonirradiated human dentin. Biomed Res Int 2015:798972. https://doi.org/10.1155/2015/798972
Vogl V, Hiller KA, Buchalla W, Federlin M, Schmalz G (2016) Controlled, prospective, randomized, clinical split-mouth evaluation of partial ceramic crowns luted with a new, universal adhesive system/resin cement: results after 18 months. Clin Oral Investig 20(9):2481–2492. https://doi.org/10.1007/s00784-016-1779-2
Milia E, Cumbo E, Cardoso RJ, Gallina G (2012) Current dental adhesives systems. A narrative review. Curr Pharm Des 18:5542–5552
Kleverlaan CJ, Feilzer AJ (2005) Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater 21:1150–1157
Kermanshahi S, Santerre JP, Cvitkovitch DG, Finery Y (2010) Biodegradation of resin–dentin interfaces increases bacterial microleakage. J Dent Res 89:996–1001. https://doi.org/10.1177/0022034510372885
Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS (2014) Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res 93:1243–1249. https://doi.org/10.1177/0022034514550039
Milia E, Pinna R, Castelli G, Bortone A, Marceddu S, Garcia-Godoy F, Gallina G (2012) TEM morphological characterization of a one-step self-etching system applied clinically to human caries-affected dentin and deep sound dentin. Am J Dent 25:321–326
Pinna R, Maioli M, Eramo S, Mura I, Milia E (2015) Carious affected dentine: its behaviour in adhesive bonding. Aust Dent J 60:276–293. https://doi.org/10.1111/adj.12309
Zhang SC, Kern M (2009) The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci 1:163–176
Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, NieuwAmerongen AV (2001) Sialometry and sialochemistry: diagnostic tools for Sjögren's syndrome. Ann Rheum Dis 60:1110–1116
Gillam DG (2013) Current diagnosis of dentin hypersensitivity in the dental office: an overview. Clin Oral Investig 17:S21–S29. https://doi.org/10.1007/s00784-012-0911-1
Funding
The authors declare that no financial relationships exist regarding any of the products involved in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The protocol and informed consent forms were approved by the Ethics Committee of the University of Sassari (no. 1000/CE).
The study followed CONSORT guidelines and was registered at the US National Institutes of Health (ClinicalTrials.gov) # NCT02766127.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinna, R., Milia, E., Usai, P. et al. Efficiency of desensitizing materials in xerostomic patients with head and neck cancer: a comparative clinical study. Clin Oral Invest 24, 2259–2269 (2020). https://doi.org/10.1007/s00784-019-03081-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03081-x