Abstract
Objectives
The objective of the study was to conduct a systematic review of literature assessing botulinum toxin type A (BoNT-A) safety and adverse effects in the treatment of myofascial pain (MFP) and trigeminal neuralgia (TN).
Materials and methods
The search for articles by two specific researchers involved the PubMed, EMBASE, Web of Science, and Scopus databases. Specific terms were used, and no publication time and language restrictions were applied. Clinical trials that investigated the effects of BoNT-A among participants with myofascial pain in masticatory muscles or trigeminal neuralgia were considered eligible for this systematic review. Data for each study were extracted and analyzed according to a PICO-like structured reading.
Results
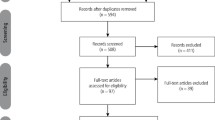
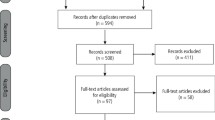
The search strategy provided 436 citations. After analysis, 16 citations were included, seven for MFP and nine for TN. In all studies, BoNT-A was well tolerated and improved pain. The most common adverse effects were temporary regional weakness, tenderness over the injection sites, and minor discomfort during chewing. Most studies reported a spontaneous resolution of adverse effect.
Conclusions
It can be concluded that BoNT-A treatment is well tolerated, since minor adverse effects were the most frequently reported; however, it is recommended that future studies aim to assess the safety and possible adverse effects of multiples applications or high doses of this treatment.
Clinical relevance
BoNT-A has been increasingly diffused in dentistry, being used for the management of masticatory myofascial pain and trigeminal neuralgia. Nonetheless, there is no consensus about its efficacy and adverse effects that could occur when this treatment is applied.

Similar content being viewed by others
References
Pellett S, Yaksh TL, Ramachandran R (2015) Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 7:4519–4563
Thenganatt MA, Fahn S (2012) Botulinum toxin for the treatment of movement disorders. Curr Neurol Neurosci Rep 12:399–409
Colhado OCG, Boeing M, Ortega LB (2009) Botulinum toxin in pain treatment. Braz J Anesthesiol 59:366–381
Dressler D, Adib Saheri F, Reis Barbosa E (2005) Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr 63:180–185
Matak I, Lackovic Z (2014) Botulinum toxin a, brain and pain. Prog Neurobiol 119–120:39–59
Lora VRMM, Clemente-Napimoga JT, Abdalla HB, Macedo CG, de la Canales Torre G, Barbosa CMR (2017) Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomadibular joint of rats. Toxicon 129:52–57
Matak I, Tékus V, Bölcskei K, Lacković Z, Helyes Z (2017) Involvement of substance P in the antinociceptive effect of botulinum toxin type A: evidence from knockout mice. Neuroscience 358:137–145
Wu C-J, Lian Y-J, Zheng Y-K, Zhang H-F, Chen Y, Xie N-C, Wang LJ (2012) Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia 32:443–450
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14:162–173
Ranoux D, Attal N, Morain F, Bouhassira D (2008) Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol 64:274–283
De la Torre Canales G, Câmara-Souza MB, do Amaral CF, Garcia RCMR, Manfredini D (2017) Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin Oral Investig
Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N et al (2014) Two doses of botulinum toxin type a for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain 15:1–6
Zúñiga C, Piedimonte F, Díaz S, Micheli F (2013) Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol 36:146–150
Bohluli B, Motamedi MHK, Bagheri SC, Bayat M, Lassemi E, Navi F, Moharamnejad N (2011) Use of botulinum toxin A for drug-refractory trigeminal neuralgia: preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111:47–50
Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT, Hirayama K (2016 Dec) Therapeutic efficacy and safety of botulinum toxin A therapy in trigeminal neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain 17(1):63
Hu Y, Guan X, Fan L, Li M, Liao Y, Nie Z, Jin L (2013) Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic review. J Headache Pain 14:72
Safarpour Y, Jabbari B (2018) Botulinum toxin treatment of pain syndromes –an evidence-based review. Toxicon. 147:120–128
Soares A, Andriolo RB, Atallah AN, da Silva EM (2014) Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst Rev (7):CD007533
Khalifeh M, Mehta K, Varguise N, Suarez-Durall P, Enciso R (2016) Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome: a systematic review and meta-analysis. J Am Dent Assoc 147(12):959–973
Turton K, Chaddock JA, Acharya KR (2002) Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci 27:552–558
Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM (2005) Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol 53:407–415
Kun-Darbois JD, Libouban H, Chappard D (2015) Botulinum toxin in masticatory muscles of the adult rat induces bone loss at the condyle and alveolar regions of the mandible associated with a bone proliferation at a muscle enthesis. Bone 77:75–82
Kim BW, Park GH, Yun WJ, Rho NK, Jang KA, Won CH, Chang SE, Chung SJ, Lee MW (2014) Adverse events associated with botulinum toxin injection: a multidepartment, retrospective study of 5310 treatments administered to 1819 patients. J Dermatol Treat 25:331–336
Rafferty KL, Liu ZJ, Ye W, Navarrete AL, Nguyen TT, Salamati A, Herring SW (2012) Botulinum toxin in masticatory muscles: short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits. Bone 50:651–662
Raphael KG, Tadinada A, Bradshaw JM, Janal MN, Sirois DA, Chan KC, Lurie AG (2014) Osteopenic consequences of botulinum toxin injections in the masticatory muscles: a pilot study. J Oral Rehabil 41(8):555–563
Lee HJ, Kim SJ, Lee KJ, Yu HS, Baik HS (2017) Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: a longitudinal study. Korean J Orthod 47(4):222–228
Ho KY, Tan KH (2007) Botulinum toxin A for myofascial trigger point injection: a qualitative systematic review. Eur J Pain 11(5):519–527
Nixdorf DR, Heo G, Major PW (2002) Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 99(3):465–473
Khawaja SN, Scrivani SJ, Holland N, Keith DA (2017) Effectiveness, safety, and predictors of response to botulinum toxin type a in refractory masticatory myalgia: a retrospective study. J Oral Maxillofac Surg 75(11):2307–2315
Kurtoglu C, Gur OH, Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H (2008) Effect of botulinum toxin- A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg 66(8):1644–1651
Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G (2008) Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio. 26(2):126–135
Abboud WA, Hassin-Baer S, Joachim M, Givol N, Yahalom R (2017) Localized myofascial pain responds better than referring myofascial pain to botulinum toxin injections. Int J Oral Maxillofac Surg 46(11):1417–1423
Ernberg M, Hedenberg-Magnusson B, List T, Svensson P (2011) Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain. 152(9):1988–1996
Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D (2012) Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio. 30(2):95–102
Piovesan EJ, Teive HG, Kowacs PA, Della Coletta MV, Werneck LC, Silberstein SD (2005) An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 65(8):1306–1308
Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N, Wu C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15:65
Türk Börü Ü, Duman A, Bölük C, Coşkun Duman S, Taşdemir M (2017) Botulinum toxin in the treatment of trigeminal neuralgia: 6-month follow-up. Medicine (Baltimore) 96(39):e8133
Caldera MC, Senanayake SJ, Perera SP, Perera NN, Gamage R, Gooneratne IK (2018) Efficacy of botulinum toxin type an in trigeminal neuralgia in a South Asian Cohort. J Neurosci Rural Pract 9(1):100–105
Liu J, Xu YY, Zhang AL, Luo WF (2018) Efficacy and safety of botulinum toxin type a in treating patients of advanced age with idiopathic trigeminal neuralgia. Pain Res Manag 7365148:5
Wu CJ, Lian YJ, Zheng YK, Zhang HF, Chen Y, Xie NC, Wang LJ (2012) Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 32(6):443–450
Shehata HS, El-Tamawy MS, Shalaby NM, Ramzy G (2013) Botulinum toxin-type A: could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 14:92
Zhang H, Lian Y, Xie N, Chen C, Zheng Y (2017) Single-dose botulinum toxin type a compared with repeated-dose for treatment of trigeminal neuralgia: a pilot study. J Headache Pain 18(1):81
Ramirez-Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z (2013) Diffusion, spread and migration of botulinum toxin. Mov Disord 28(13):1775–1783
Kim KS, Byun YS, Kim YJ, Kim ST (2009) Muscle weakness after repeated injection of botulinum toxin type A evaluated according to bite force measurement of human masseter muscle. Dermatol Surg 35(12):1902–1906
Park HU, Kim BI, Kang SM, Kim ST, Choi JH, Ahn HJ (2013) Changes in masticatory function after injection of botulinum toxin type A to masticatory muscles. J Oral Rehabil 40(12):916–922
Tsai CY, Lin YC, Su B, Yang LY, Chiu WC (2012) Masseter muscle fibre changes following reduction of masticatory function. Int J Oral Maxillofac Surg 41(3):394–399
Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W (2011) Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech 44(1):39–44
Gedrange T, Gredes T, Spassov A, Mai R, Kuhn DU, Dominiak M, Kunert-Keil C (2013) Histological changes and changes in the myosin mRNA content of the porcine masticatory muscles after masseter treatment with botulinum toxin A. Clin Oral Investig 17(3):887–896
Raadsheer MC, van Eijden TM, van Ginkel FC, Prahl-Andersen B (1999) Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. J Dent Res 78(1):31–42
Matthys T, Ho Dang HA, Rafferty KL, Herring SW (2015) Bone and cartilage changes in rabbit mandibular condyles after 1 injection of botulinum toxin. Am J Orthod Dentofac Orthop 148(6):999–1009
Funding source
Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (number 2017/21674-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix. CASP, Critical Appraisal Skills Programme
Appendix. CASP, Critical Appraisal Skills Programme
To access all 12 items of CASP quality assessment for cohort studies, click the following link:
https://casp-uk.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf
Rights and permissions
About this article
Cite this article
De la Torre Canales, G., Poluha, R.L., Lora, V.M. et al. Botulinum toxin type A applications for masticatory myofascial pain and trigeminal neuralgia: what is the evidence regarding adverse effects?. Clin Oral Invest 23, 3411–3421 (2019). https://doi.org/10.1007/s00784-019-03026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03026-4