Abstract
Objectives
There is a long history of studies into cystic lesions of the jaws. However, there is little data on postoperative bone regeneration. The aim of the study was to calculate the size of the residual bone lesions after cystostomies and cystectomies to predict the postoperative bone healing.
Material and methods
This retrospective study focused on patients undergoing cystectomy (n = 114) or cystostomy (n = 86), who were treated for cystic lesions between 2010 and 2015. New formulas were developed to measure the decrease in lesion sizes using the craniocaudal and mesiodistal diameters from preoperative as well as postoperative radiographs (panoramic radiographs, CT scans, CBCT). With these predictive models (calculated by linear regression analyses), it is possible to calculate how the lesions would shrink using initial size of the cyst and time after surgery for both dimensions.
Results
After cystostomy, the mesiodistal size was initial size 6.0 + 0.7 × initial size − 9.4 × time. The craniocaudal size was 5.1 + 0.7 × initial size − 7.3 × time. After cystectomy, the mesiodistal size was 0.4 + 0.7 × initial size − 8.4 × time. The craniocaudal size was 0.4 + 0.5 × initial size − 6.4 × time (R2 = 0.59 and 0.67, respectively).
Conclusion
These formulas are suggested to be used to calculate residual size of bone lesion after cystostomy and cystectomy to predict postoperative bone healing.
Clinical relevance
The formulas are based on deliberately simple measurement using only panoramic radiographs to encourage their application in daily clinical practice.
Similar content being viewed by others
Introduction
Cystic lesions of the jaws are common pathologies of the oral region. As most cysts grow without symptoms, they can cause major bone defects and are usually discovered unintentionally in the course of routine radiographs. Jaw cysts develop either from an epithelium with an odontogenic origin or from non-odontogenic origin (odontogenic cysts and non-odontogenic cysts), or they have no epithelial lining at all (i.e. aneurysmal bone cyst, simple bone cyst). Some diseases that mimic cystic lesions, such as ameloblastoma and central giant cell granuloma, are not categorised histologically as cysts but as benign odontogenic tumours with differing treatment options [1, 2]; these were not included in this study.
Various therapies for treating cysts of the jaws were described more than a hundred years ago by Partsch et al., and they are still used today, with only minor modifications [3,4,5]. The two most common treatments are primary enucleation and mucosal closure of the defect called “cystectomy” [6, 7] or decompression therapy called “cystostomy” [8, 9] using obturators, decompression tubes, cannulated screws, or the marsupialization of the cyst followed by a secondary enucleation.
The most suitable treatment should be chosen based on the size of the cyst and its proximity to the inferior alveolar nerve and teeth. A review done by Wakolbinger et al. [3] in 2015 shows that cysts with a diameter of up to 1.5 cm can safely be enucleated immediately. Primary enucleation of larger cysts, however, can pose an increased risk of damage to the inferior alveolar nerve and to the teeth resulting in sensory deficits, the devitalization of teeth or in bone fractures [10]. Wakolbinger’s findings are also reflected in recent literature, which show a trend towards favouring decompression therapy of large cysts, since decompression therapy has a lower risk of complications [9, 11,12,13].
Despite the ubiquity of jaw cysts in clinical practice, the numerous retrospective analyses that have been done lack long-term follow-ups in postoperative bone healing [3, 7, 14].
Benign jaw cysts are commonly treated by cystectomy or cystostomy (marsupialization) with or without secondary cystectomy. The expected time to radiographic resolution is difficult to predict. Currently, there are no objective methods for doing so.
The ability to correctly predict the amount of time needed for jaw bone to heal would greatly assist surgeons in choosing the best time to perform a secondary enucleation after cystostomy, or implantation following primary enucleation. It can also simply help them answer their patients’ questions. The aim of this study is to perform a retrospective analysis of benign jaw cysts treated with cystectomy or cystostomy so as to develop a formula for predicting expected size reduction over time.
Materials and methods
Study design and data collection
A retrospective study was carried out, in accordance with the Declaration of Helsinki, after receiving approval from the ethical committee of the Medical University of Vienna, Austria (1936/2015) [15]. The results are reported according to the STROBE criteria [16]. The medical records of all patients with cystic lesions were reviewed, who underwent an operation at the Department of Oral and Maxillofacial Surgery of the Medical University of Vienna, between January 2010 and January 2015. Patients were reviewed that were diagnosed with odontogenic, non-odontogenic developmental, or simple bone cysts, in accordance with the World Health Organisation’s updated classification of 2017 [17]. This recent WHO update reclassifies the odontogenic keratocyst (OKC) as an odontogenic cyst [18]. Gender, age, histological diagnosis, therapy, and follow-up data were studied. The radiological data from all available digital panoramic radiographs (Px), CT scans (CT), and cone beam CT (CBCT) deal with the cyst’s location in the jaw, and its size. All patients with a minimum follow-up of 1 month were included to analyse the size of the residual lesion after treatment. All cases with insufficient documentation, analogue radiographs, and histological results other than those described above were excluded.
Treatment protocol and surgical technique
During the research period, there was no clearly defined treatment protocol for jaw cysts at the department. It was solely the surgeon’s decision whether to recommend doing a cystostomy followed by a secondary cystectomy or to perform a primary cystectomy without decompression therapy. Cystostomies were performed by opening the cyst, taking a biopsy, and inserting a plastic tube with a diameter of 5 mm to connect the lumen of the cyst with the oral cavity, as described by Enislidis et al. [8]. The patients were instructed to rinse this tube at least twice a day with a sterile saline solution using a syringe and a blunt cannula. Before the final step, a cystectomy, the surgeon determined the duration of the decompression therapy based on several radiological check-ups.
Image acquisition and measurements
A total of 1222 Px and 625 CT scans were taken; all of which were analysed for this retrospective study. We used a Px as the main radiographic follow-up, and three-dimensional imaging was performed preoperatively for further diagnosis and, in some cases, also for follow-ups. If the Px did not offer decisive information with regard to the degree of bone healing, a CT scan or CBCT was performed (e.g. osseous structures separating the cyst and the inferior alveolar neurovascular bundle). The images were transferred to a private workstation at the Department of Oral and Maxillofacial Surgery from the archives of the Department of Radiology as DICOM-files. All radiographs were anonymized before the measurements and provided with consecutive patient numbers.
All the measurements were taken using the open source program OsiriX Software (version 9.0, Pixmeo SARL, Bernex, Switzerland). Panoramic radiographs were performed using a panoramic X-ray machine (Orthophos XG 5, Dentsply Sirona, Salzburg, Austria, 64 kV, 8 mA), 3D examinations on a 64-multidector row CT scanner (Philips Brilliance CT 64, Philips Healthcare, Eindhoven, Netherlands, 120 kV, 30 mA, field of view 130–220 mm), and on a CBCT scanner (KaVo 3D eXam, KaVo Dental Excellence, Biberbach, Germany, 120 kV, 5 mA, voxel size 0,4 mm, field of view of up to 160 mm × 130 mm).
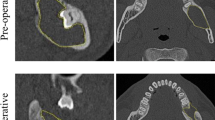
The maximum extension of the cyst was defined either in the craniocaudal (CC) or in the mesiodistal (MD) length, in accordance with the recent proposal of Anavi et al. [12]. A second measurement was carried out at a 90° angle to the first axis. The measuring was done between the first sclerotic borders of the radiolucent lesions; an example of these measurements is given in Fig. 1. The images were measured according to instructions given by a board-certified radiologist. One author (C.S.) did all the measurements. Where there were statistical outliers (defined as an increased cyst size after treatment compared to previous measurements), the measurement was checked and corrected if inaccurate. The measurements were repeated for control purposes and corrected if inaccurate. Four regions of the jaw were defined: anterior maxilla (region between the upper canines), posterior maxilla (first premolar to the maxillary tuberosity), anterior mandible (region between the lower canines), and posterior mandible (first premolar to the mandibular ramus).
Example of measurements and follow-up of a cystic lesion. Left Px: preoperatively, measured size 30.6 mm (md) and 37.2 (cc). Middle Px: follow-up after 6.5 months, measured size 24.1 mm (md) and 26.6 (cc); calculated size 22.3 (md) and 26.6 (cc); Right Px: follow-up after 39.2 months, measured size 0 mm (md) and 0 mm (cc); calculated size − 3.3 mm(md) and 3.7 mm (cc)
Statistical methods
Data was recorded using Microsoft Excel (version 14.6.4., Microsoft, Redmond, WA, USA). Statistical analysis was performed using the open source program “R” (version 3.4.2). Statistical significance was set at a p value < 0.05. For the demographic data age, initial cc and md sizes were checked for normal distribution by Shapiro-Wilk test and QQ plots; in case of normal distribution, a t test was used. Shrinkage was described in linear regression models, and md and cc size were regressed by the initial size and time. It was assumed that shrinkage follows a linear time-dependent bone healing. Wald’s test was used to reveal coefficients significantly different to zero.
Data correction of Px data
The scaling of the radiographic modalities was done using non-uniform magnification factors, which were computed in linear regression models for both lengths. If both the Px and the CT of a certain patient were recorded within 2 weeks preoperatively, with no relevant change in lesion size, both measurements were added to the linear regression model. There were 107 patients who had both a Px and a CT scan done within 2 weeks, preoperatively. Those radiographic records were used for linear regression models for the mesiodistal and craniocaudal size to scale the Px data to CT sizes. Px data from all the other included patients was corrected by these two linear regression models to scale their Px data.
Cyst size model
It was calculated how the cysts would shrink using the linear regression models for the mesiodistal and craniocaudal length for each of the two treatment methods. Wald’s tests were used to analyse effects of time, initial cyst size, and the interaction of initial size and time in years.
Results
Study sample and tests for bias
From January 2010 to January 2015, 822 patients were treated with cystic lesions of the jaw at the Department of Oral and Maxillofacial Surgery of the Medical University of Vienna. These 822 patients met the inclusion criteria (cystic lesions, which were treated by cystostomy or cystectomy). Out of these 822 patients, 190 had to be excluded due to incomplete medical records or to histopathological diagnosis other than a cyst, and 4 patients with odontogenic keratocysts showed a recurrence (6.06%) and had to be excluded as well. Another 428 patients had to be excluded due to incomplete radiological follow-up data, leaving 200 patients that could be used for the study according to the inclusion criteria. To detect a potential bias, the excluded 428 patients were compared to the included 200 patients regarding all relevant initial parameters (see Table 1). This analysis showed that the study sample consisted of cysts that were significantly larger and had a higher percentage of odontogenic keratocysts (23.5%) with a higher risk of relapse than the excluded patients (4.4%). Maxillary cysts were either located in the anterior (n = 31, 15.5%) or the posterior maxilla (n = 21, 10.5%). In the lower jaw, the cysts were found more often in the posterior (n = 134, 67.0%) than in the anterior mandibular region (n = 14, 7.0%). The residual lesion size was a result of the treatment option, the initial cyst size, and when the measurements were taken. Therefore, the regression models were based on data of 86 (out of 123) patients who were treated by cystostomy, as well as on data of 114 (out of 505) patients who had a cystectomy. A total of 1222 Px, 625 CT scans, and 10 CBCTs were measured and analysed. 15.3% of the patients were followed-up to a residual size of up to 10% of the initial size, 36.0 to 20% of the initial size, and 42.3% to one-third of the initial size.
Correction of panoramic radiograph data
Since Px measurements differ from CT measurements, it was necessary to rescale the Px measurements to the CT scan. This was done by linearly regressing the measurements in the craniocaudal and mesiodistal dimensions.
In 107 cases, both mesiodistal and craniocaudal distances were measured in a Px and a CT scan, which were recorded within a time span of 2 weeks. These cases were used to correct the distortion of Px, as no relevant changes in lesion size were to be expected during this time. The regression analyses showed a multiple R2 of 0.92 for both dimensions. The regression models revealed a magnification factor of 1.25 in mesiodistal and a magnification factor of 1.16 in craniocaudal dimension. The regression models are as follows:
Analysis of residual cyst size
A total of 200 patients were included: 86 had a cystectomy and 114 had a cystostomy. The histological diagnosis, age, gender, and shrinkage behaviour had no significant influence on the residual cyst size. The relevant initial parameters are shown in Table 2.
Residual cyst size after cystostomy
These analyses were based on the data of 86 cystostomy patients with a mean follow-up of 8.4 ± 7.7 months. The regression analyses for residual cyst size after cystostomy showed a multiple R2 of 0.67 for the mesiodistal and 0.64 for the craniocaudal dimension. Both dimensions depended significantly on the intercept, initial size, and time (in years) since operation (pWald for all parameters were < 0.001). The linear regression models for residual size of the bone lesion after cystostomy are shown in the following equations (M = mesiodistal size in mm at the time of follow-up; m = initial mesiodistal size in mm; C = craniocaudal size in mm at the time of follow-up; c initial craniocaudal size in mm; t = time of follow-up in years).
Residual cyst size after cystectomy
These analyses were based on the data of 114 cystectomy patients with a mean follow-up of 11.6 ± 11.8 months. The regression analyses showed a multiple R2 of 0.62 for the mesiodistal and 0.59 for the craniocaudal dimension. Both dimensions depended significantly on initial size and time (in years) since operation (pWald < 0.001), while the intercept was significant for the MD size only (pWald < 0.001; pWald = 0.746 for CC size). The linear regression models for residual size of the bone lesion after cystectomy are shown in the following equation (M = mesiodistal size in mm at the time of follow-up; m = initial mesiodistal size in mm; C = craniocaudal size in mm at the time of follow-up; c initial craniocaudal size in mm; t = time of follow-up in years).
Discussion
The aim of the study was to calculate the shrinking of residual bone lesions after cystostomies and cystectomies to predict the postoperative bone healing. This paper is—to our knowledge—the first to provide a simple mathematical model for calculating time for postoperative bone regeneration of cystic lesions.
A wide range of studies has reported the different indications for immediate cystectomy and cystostomy, as well as their respective advantages and disadvantages [3, 7, 19, 20]. Data on bone healing after surgery and the shrinkage behaviour of bone lesions after a cystostomy is, however, still limited [12, 21,22,23]. Yet, these shrinkage rates are needed to predict complete bone healing and to determine reasonable follow-up intervals during treatments.
The shrinkage behaviour of cystic lesions was dependent on the initial cyst size and the time after surgery, which could be shown in previous studies [11, 21].
There was no statistically significant association between the shrinkage rate and the underlying histology of the lesion. Several different cysts were included, with different clinical natural histories, and no association between the shrinkage and the histology was found in these patient collectives, even in subset analysis of the more common cysts.
The finding is in line with a study by Anavi et al. who analysed 73 cystic lesions treated by cystostomy [12]. They did not discover a difference in the histological types, and they reported reduction rates of the lesion area between 78.85% and 85.64% depending on the age of the patient and decompression time, with a mean decompression time of 9.2 ± 5.2 months [12].
Gao et al., on the other hand, reported a decrease in lesion size after decompression therapy in the form of absolute volumetric changes with a reduction of the radiolucent area between 2.71cm2 and 3.37cm2 per month [14]. They showed a difference in the histological types regarding the bone healing.
Song et al. reported relative reductions in volume between 48.5% and 56.9% during a mean decompression treatment period of 194 up to 462 days [21]. None of these studies, however, provide a mathematical model to predict the residual size of the lesion size given the initial size and treatment.
While numerous methods of measuring cystic lesions have been described [3, 21, 24,25,26], the reduction of the defect after surgery is increasingly measured by segmentation of 3D data sets [13, 21]. Some of these methods are time-consuming and rather complicated, although they are becoming faster and easier to use. In contrast to this, the measurements were kept deliberately simple to encourage their application in daily clinical practice. While being easy to use, the formula allows for an accurate and precise prediction of the amount of time needed for bone regeneration after both cystostomy and cystectomy. Furthermore, the linear regression model to scale the Px data to 3D imagings showed a multiple R2 of 0.92. Therefore, the additional value of 3D imaging is less in the assessment of the size of the cyst than in the assessment of the margins of the cyst and near anatomical structures.
In the future, all initial radiological examinations will be three-dimensional and, if necessary, follow-up will continue to be performed mainly with Px.
The linear regression models for the residual size of the bone lesion revealed high coefficients of determination with R2 values between 0.59 and 0.67. Due to different methods of measurement, the mathematical model presented in this study cannot be compared directly to the shrinkage behaviour reported in previous studies.
The initial cystic lesions of patients who were treated by cystostomy were larger than those of patients who were treated by cystectomy, which confirms the indication of cystostomy in large jaw cysts.
One of the limitations of the findings is that the included and excluded patients showed significant differences in how often the cyst occurred in the four specified regions, in histopathological diagnosis, and in size. This can be ascribed to the higher percentage of included patients with odontogenic keratocysts. Odontogenic keratocysts occur most frequently in the posterior region of the mandible [27, 28], which explains the difference in cyst localization between included and excluded patients. At the department, patients with odontogenic keratocysts have radiological examinations on a regular basis to reveal any recurrences of the lesion in a long-term follow-up period. This long-term follow-up period explains the high amount of included odontogenic keratocysts.
Another limitation is a possible underestimation of the effect of the decompression therapy using the formula, as patients with lower rates of bone healing may have had a longer time of decompression therapy before secondary cystectomy.
A third limitation is the disadvantage of using panoramic radiographs, as there is a certain degree of magnification and distortion of the depicted structures—depending on the location of the structure, which make measurements in Px inaccurate and there is the possibility of positioning errors [29]. Scaling the measured values of the panoramic radiographs using the regression analyses to produce case-specific and therefore non-uniform magnification factors reduces the magnification and distortions to a minimum.
A final limitation of this retrospective study is the relatively short average follow-up of cystostomy and cystectomy patients (0.97 years and 0.70 years, respectively).
Nevertheless, the formula is suggested to surgeons because it is mathematically simple, not time-consuming, and accurate.
The findings of the study contribute to a better understanding of the residual lesion size at any given time after cystostomy and improve the prediction of the time needed for bone regeneration after cystectomy. Future prospective studies with longer follow-up will be needed to allow for more detailed conclusions.
References
Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO (2017) Ameloblastoma: current etiopathological concepts and management. Oral Dis 24:307–316. https://doi.org/10.1111/odi.12646
Meshram M, Sagarka L, Dhuvad J, Anchlia S, Vyas S, Shah H (2017) Conservative management of unicystic ameloblastoma in young patients: a prospective single-center trial and review of literature. J Maxillofac Oral Surg 16(3):333–341. https://doi.org/10.1007/s12663-016-0987-2
Wakolbinger R, Beck-Mannagetta J (2015) Long-term results after treatment of extensive odontogenic cysts of the jaws: a review. Clin Oral Investig 20:15–22. https://doi.org/10.1007/s00784-015-1552-y
Partsch C (1892) Über Kiefercysten. Dtsch Monatsschr Zahnheilk 10:271–304
Partsch C (1910) Zur Behandlung der Kieferzysten. Dtsch Monatsschr Zahnheilk 28:252–260
Ihan Hren N, Miljavec M (2008) Spontaneous bone healing of the large bone defects in the mandible. Int J Oral Maxillofac Surg 37(12):1111–1116. https://doi.org/10.1016/j.ijom.2008.07.008
Ettl T, Gosau M, Sader R, Reichert TE (2012) Jaw cysts - filling or no filling after enucleation? A review. J Craniomaxillofac Surg 40(6):485–493. https://doi.org/10.1016/j.jcms.2011.07.023
Enislidis G, Fock N, Sulzbacher I, Ewers R (2004) Conservative treatment of large cystic lesions of the mandible: a prospective study of the effect of decompression. Br J Oral Maxillofac Surg 42(6):546–550. https://doi.org/10.1016/j.bjoms.2004.06.020
Allon DM, Allon I, Anavi Y, Kaplan I, Chaushu G (2015) Decompression as a treatment of odontogenic cystic lesions in children. J Oral Maxillofac Surg 73(4):649–654. https://doi.org/10.1016/j.joms.2014.10.024
Liang YJ, He WJ, Zheng PB, Liao GQ (2015) Inferior alveolar nerve function recovers after decompression of large mandibular cystic lesions. Oral Dis 21(5):674–678. https://doi.org/10.1111/odi.12338
Asutay F, Atalay Y, Turamanlar O, Horata E, Burdurlu MC (2016) Three-dimensional volumetric assessment of the effect of decompression on large mandibular odontogenic cystic lesions. J Oral Maxillofac Surg 74(6):1159–1166. https://doi.org/10.1016/j.joms.2015.12.010
Anavi Y, Gal G, Miron H, Calderon S, Allon DM (2011) Decompression of odontogenic cystic lesions: clinical long-term study of 73 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(2):164–169. https://doi.org/10.1016/j.tripleo.2010.09.069
Park HS, Song IS, Seo BM, Lee JH, Kim MJ (2014) The effectiveness of decompression for patients with dentigerous cysts, keratocystic odontogenic tumors, and unicystic ameloblastoma. J Korean Assoc Oral Maxillofac Surg 40(6):260–265. https://doi.org/10.5125/jkaoms.2014.40.6.260
Gao L, Wang XL, Li SM, Liu CY, Chen C, Li JW, Yan XJ, Zhang J, Ren WH, Zhi KQ (2014) Decompression as a treatment for odontogenic cystic lesions of the jaw. J Oral Maxillofac Surg 72(2):327–333. https://doi.org/10.1016/j.joms.2013.07.035
World Medical A (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194. https://doi.org/10.1001/jama.2013.281053
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Adel K El-Naggar JKC, Jennifer R Grandis, Takashi Takata, Pieter J Slootweg (2017) WHO classification of head and neck tumours. Fourth edition. IARC WHO Classification of Tumours, No 9
Wright JM, Vered M (2017) Update from the 4th edition of the World Health Organization Classification of Head and Neck Tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol 11(1):68–77. https://doi.org/10.1007/s12105-017-0794-1
Al-Moraissi EA, Dahan AA, Alwadeai MS, Oginni FO, Al-Jamali JM, Alkhutari AS, Al-Tairi NH, Almaweri AA, Al-Sanabani JS (2017) What surgical treatment has the lowest recurrence rate following the management of keratocystic odontogenic tumor?: a large systematic review and meta-analysis. J Craniomaxillofac Surg 45(1):131–144. https://doi.org/10.1016/j.jcms.2016.10.013
Manor E, Kachko L, Puterman MB, Szabo G, Bodner L (2012) Cystic lesions of the jaws - a clinicopathological study of 322 cases and review of the literature. Int J Med Sci 9(1):20–26
Song IS, Park HS, Seo BM, Lee JH, Kim MJ (2015) Effect of decompression on cystic lesions of the mandible: 3-dimensional volumetric analysis. Br J Oral Maxillofac Surg 53(9):841–848. https://doi.org/10.1016/j.bjoms.2015.06.024
Lizio G, Sterrantino AF, Ragazzini S, Marchetti C (2013) Volume reduction of cystic lesions after surgical decompression: a computerised three-dimensional computed tomographic evaluation. Clin Oral Investig 17(7):1701–1708. https://doi.org/10.1007/s00784-012-0869-z
Jeong HG, Hwang JJ, Lee SH, Nam W (2017) Effect of decompression for patients with various jaw cysts based on a three-dimensional computed tomography analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 123(4):445–452. https://doi.org/10.1016/j.oooo.2016.11.012
Nakamura N, Mitsuyasu T, Mitsuyasu Y, Taketomi T, Higuchi Y, Ohishi M (2002) Marsupialization for odontogenic keratocysts: long-term follow-up analysis of the effects and changes in growth characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94(5):543–553
Vallaeys K, Kacem A, Legoux H, Le Tenier M, Hamitouche C, Arbab-Chirani R (2015) 3D dento-maxillary osteolytic lesion and active contour segmentation pilot study in CBCT: semi-automatic vs manual methods. Dentomaxillofac Radiol 44(8):20150079. https://doi.org/10.1259/dmfr.20150079
Safi AF, Grandoch A, Dreiseidler T, Nickenig HJ, Zoller J, Kreppel M (2017) Can cyst volume be used to stratify risk of complications following cyst defect reconstruction with iliac crest graft? J Craniomaxillofac Surg 45:1165–1169. https://doi.org/10.1016/j.jcms.2017.05.010
Zecha JA, Mendes RA, Lindeboom VB, van der Waal I (2010) Recurrence rate of keratocystic odontogenic tumor after conservative surgical treatment without adjunctive therapies - a 35-year single institution experience. Oral Oncol 46(10):740–742. https://doi.org/10.1016/j.oraloncology.2010.07.004
Boffano P, Ruga E, Gallesio C (2010) Keratocystic odontogenic tumor (odontogenic keratocyst): preliminary retrospective review of epidemiologic, clinical, and radiologic features of 261 lesions from University of Turin. J Oral Maxillofac Surg 68(12):2994–2999. https://doi.org/10.1016/j.joms.2010.05.068
Devlin H, Yuan J (2013) Object position and image magnification in dental panoramic radiography: a theoretical analysis. Dentomaxillofac Radiol 42(1):29951683. https://doi.org/10.1259/dmfr/29951683
Acknowledgements
Open access funding provided by Medical University of Vienna. Thanks to Salwa de Vree for diligently editing the manuscript and to Laura Holzer-Frühwald for many dedicated hours of instructions on radiology measurements. Thanks to Christos Perisanidis for making preliminary corrections and Friederike Herden for the help with the clinical database. Thanks are also given to the Department of Oral & Maxillofacial Surgery, Medical University of Vienna, Austria, for the institutional support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The Institutional Review Board of the Medical University of Vienna, Austria, has reviewed the research proposal and has granted us permission to analyse the data (protocol-no: 1932/2016).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sacher, C., Holzinger, D., Grogger, P. et al. Calculation of postoperative bone healing of cystic lesions of the jaw—a retrospective study. Clin Oral Invest 23, 3951–3957 (2019). https://doi.org/10.1007/s00784-019-02826-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02826-y