Abstract
Objectives
The present study aimed to evaluate the efficacy of α-bisabolol (BISA)-based mouthwashes in the oral hygiene of patients submitted to oral and maxillofacial surgery.
Materials and methods
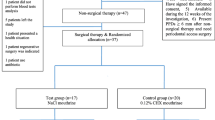
A randomized, controlled, triple-blind clinical trial was conducted with 30 patients, undergoing oral and maxillofacial surgery. Three types of mouthwashes were developed, based at 0.12% chlorhexidine, 0.5% BISA, and 0.12% chlorhexidine + 0.5% BISA. The patients were evaluated in the preoperative and postoperative period, divided into three groups according to the mouthwash to be used. In the postoperative period, the oral hygiene quality of the patients was evaluated through the simplified oral hygiene index; the healing of the wounds was evaluated observing the presence of suture dehiscence and/or infection, and the pain was established using the Visual Analogue Scale. The antiseptic effect of the mouthwashes was evaluated in vitro.
Results
There were no differences in the efficacy of BISA-containing mouthwashes for oral hygiene, healing, and pain, compared to chlorhexidine based at 0.12%. There were no differences in the antiseptic activity of chlorhexidine and chlorhexidine + α-bisabolol-based mouthwashes.
Conclusion
The results indicate that BISA-based mouthwashes have clinical efficacy, in the improvement of oral hygiene and wound healing, as well as in the reduction of postoperative pain.
Clinical relevance
Considering that BISA has analgesic, antimicrobial, and anti-inflammatory properties, it is relevant to evaluate the efficacy of BISA-based mouthwashes in the oral hygiene of patients undergoing oral and maxillofacial surgery, seeking a better postoperative recovery.


Similar content being viewed by others
References
Dalben GDS et al (2008) Evaluation of sutures after immersion in nonalcoholic benzydamine hydrochloride mouthrinse by scanning electron microscopy. Clin Oral Investig 12(3):287–290
Potera C (1999) Forging a link between biofilms and disease. Science 283(5409):1837–1839
Trimbos J, Brohim R, Van Rijssel E (1989) Factors relating to the volume of surgical knots. Int J Gynecol Obstet 30(4):355–359
Andrade IP et al (2011) Concentração inibitória mínina de antissépticos bucais em microorganismos da cavidade oral. Revista Brasileira de Pesquisa em Saúde/Brazilian Journal of Health Research
Bugno A et al (2006) Enxaguatórios bucais: avaliação da eficácia antimicrobiana de produtos comercialmente disponíveis. Revista do Instituto Adolfo Lutz (Impresso) 65(1):40–45
Bauroth K et al (2003) The efficacy of an essential oil antiseptic mouthrinse vs. dental floss in controlling interproximal gingivitis: a comparative study. J Am Dent Assoc 134(3):359–365
Brunke E.-J. and Hammerschmidt F.-J (1985) Constituents of the essential oil of Salvia stenophylla—first identification of (+)-epi-α-bisabolol in nature, in Essential Oils and Aromatic Plants, Springer. p. 145–150
Jakovlev V, Isaac O, Thiemer K, Kunde R (1979) Pharmacological investigations with compounds of chamomile ii. New investigations on the antiphlogistic effects of (-)-alpha-bisabolol and bisabolol oxides (author’s transl). Planta Med 35(2):125–140
Jakovlev V, Von Schlichtegroll A (1969) On the inflammation inhibitory effect of (-)-alpha-bisabolol, an essential component of chamomilla oil. Arzneimittelforschung 19(4):615–616
Kamatou GP, Viljoen AM (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87(1):1–7
Cavalieri E, Mariotto S, Fabrizi C, de Prati AC, Gottardo R, Leone S, Berra LV, Lauro GM, Ciampa AR, Suzuki H (2004) α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem Biophys Res Commun 315(3):589–594
Leite GdO et al (2011) (−)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia 82(2):208–211
Maurya AK et al (2014) α-(−)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol 15(2):173–181
Melo LT, Duailibe MAB, Pessoa LM, da Costa FN, Vieira-Neto AE, de Vasconcellos Abdon AP, Campos AR (2017) (−)-α-Bisabolol reduces orofacial nociceptive behavior in rodents. Naunyn Schmiedeberg’s Arch Pharmacol 390(2):187–195
Brasil, Farmacopeia Brasileira (2010) Agência Nacional de Vigilância Sanitária. Editor, Anvisa ^ eBrasília Brasília
Fones, A.C. and E.C. Kirk, Mouth hygiene, a course of instruction for dental hygienists: a text-book containing the fundamentals for prophylactic operators. 1916: Lea & Febiger
Heitz F, Heitz-Mayfield L, Lang N (2004) Effects of post-surgical cleansing protocols on early plaque control in periodontal and/or periimplant wound healing. J Clin Periodontol 31(11):1012–1018
Greene JG, Vermillion JR (1964) The simplified oral hygiene index. J Am Dent Assoc 68(1):7–13
Souza F, Souza R, Moraes  (2016) Incorporation and release kinetics of alpha-bisabolol from PCL and chitosan/guar gum membranes. Braz J Chem Eng 33(3):453–467
Brasil, Resolução n° 466/12 sobre pesquisa envolvendo seres humanos, Ministério da Saúde : Conselho Nacional de Saúde, Editor. 2012, Ministério da Saúde Brasília
Teixeira GFD, Vieira-Neto AE, da Costa FN, e Silva ARA, Campos AR (2017) Antinociceptive effect of (-)-α-bisabolol in nanocapsules. Biomed Pharmacother 91:946–950
Barreto RS et al (2016) Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl.(Lamiaceae) essential oil and (−)-α-bisabolol, its main compound, in mice. J Ethnopharmacol 191:9–18
Ortiz MI, Fernández-Martínez E, Soria-Jasso LE, Lucas-Gómez I, Villagómez-Ibarra R, González-García MP, Castañeda-Hernández G, Salinas-Caballero M (2016) Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed Pharmacother 78:248–256
Aron de Miranda HA et al (2010) Evaluation of the sesquiterpene (−)-α-bisabolol as a novel peripheral nervous blocker. Neurosci Lett 472(1):11–15
Simões RCS et al (2011) Avaliação in vitro da atividade antimicrobiana de enxaguatórios bucais. Rev bras odontol 68(1):91
Forrer M, Kulik EM, Filippi A, Waltimo T (2013) The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch Oral Biol 58(1):10–16
Santos NOd et al (2015) Assessing the chemical composition and antimicrobial activity of essential oils from Brazilian plants—Eremanthus erythropappus (Asteraceae), Plectrantuns barbatus, and P. amboinicus (Lamiaceae). Molecules 20(5):8440–8452
de Lima Pérez ALA et al (2011) Atividade Antifúngica de Antissépticos Bucais sobre candida spp. Revista Brasileira de Ciências da Saúde 15(1):69–74
Defever K et al (1982) Candida albicans resistance to 5-fluorocytosine: frequency of partially resistant strains among clinical isolates. Antimicrob Agents Chemother 22(5):810–815
Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39(9):2128–2131
Fischman SL (1986) Current status of indices of plaque. J Clin Periodontol 13(5):371–374
Batista ALA, Diógenes Alves Uchôa Lins R, de Souza Coelho R, do Nascimento Barbosa D, Moura Belém N, Alves Celestino FJ (2014) Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complement Ther Clin Pract 20(1):93–98
Silveira J, Oliveira Vd, Padilha WWN (2002) Avaliação da redução do índice de placa visível e do índice de sangramento gengival em uma prática de promoção de saúde bucal com crianças. Pesqui Odontol Bras 16(2):169–174
Hammad H, Hammad MM, Abdelhadi IN, Khalifeh MS (2011) Effects of topically applied agents on intra-oral wound healing in a rat model: a clinical and histomorphometric study. Int J Dent Hyg 9(1):9–16
Jesudasan JS, Wahab PA, Sekhar MM (2015) Effectiveness of 0.2% chlorhexidine gel and a eugenol-based paste on postoperative alveolar osteitis in patients having third molars extracted: a randomised controlled clinical trial. Br J Oral Maxillofac Surg 53(9):826–830
Glowania H, Raulin C, Swoboda M (1987) Effect of chamomile on wound healing—a clinical double-blind study. Zeitschrift fur Hautkrankheiten 62(17):1262 1267-71
Kim S, Jung E, Kim JH, Park YH, Lee J, Park D (2011) Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264. 7 macrophages. Food Chem Toxicol 49(10):2580–2585
Golan DE, Tashjian AH, Armstrong EJ (2012) Princípios de farmacologia. Grupo Gen-Guanabara Koogan
Foulkes D (1973) Some toxicological observations on chlorhexidine. J Periodontal Res 8(s12):55–60
Bhatia S et al (2008) Fragrance material review on α-bisabolol. Food Chem Toxicol 46(11):S72–S76
Ensina LF et al (2009) Reações de hipersensibilidade a medicamentos. Rev bras alerg imunopatol 32(2):42–47
Bernd LAG (2005) Alergia a medicamentos. Rev bras alerg imunopatol 28(3):125–132
US Food & Drug Administration, FDA Drug Safety Communication: FDA warns about rare but serious allergic reactions with the skin antiseptic chlorhexidine gluconate. US Department of Health & Human Services, 2017
Jacob SE, Hsu JW (2010) Reactions to Aquaphor®: is Bisabolol the culprit? Pediatr Dermatol 27(1):103–104
Jacob SE, Matiz C, Herro EM (2011) Compositae-associated allergic contact dermatitis from Bisabolol. Dermatitis 22(2):102–105
Pastor N, Silvestre JF, Mataix J, Lucas A, Pérez M (2008) Contact cheilitis from bisabolol and polyvinylpyrrolidone/hexadecene copolymer in lipstick. Contact Dermatitis 58(3):178–179
Wilkinson S, Hausen B, Beck M (1995) Allergic contact dermatitis from plant extracts in a cosmetic. Contact Dermatitis 33(1):58–59
Junior VFV, Pinto AC, Maciel MAM (2005) Plantas medicinais: cura segura. Química nova 28(3):519–528
Castilho AR, Murata RM, Pardi V (2007) Produtos Naturais em Odontologia. Revista Saúde-UNG 1(1):11–19
Acknowledgments
The authors are thankful to the support and partnership of the Fortaleza’s Holy House of Mercy (Santa Casa da Misericórdia de Fortaleza) for having accepted and given unconditional and essential support for this research. Without this partnership, the recruitment and clinical evaluation of patients, a fundamental part of our research, would not be possible. The authors also thank the Staff of the Department of Maxillofacial Surgery of this Hospital, Dr. José Maria Sampaio Menezes Junior, Dr. Vera Araújo Magalhães, Dr. Carla Welch da Silva, Dr. Eymard Vieira Borges, Dr. George Matos Ferreira Gomes Junior, Dr. Jonas Ferreira Maciel Gusmão, Dr. Paulo Henrique Rodrigues Carvalho, and Dr. Stélio da Conceição Araújo Neto, for their support during data collection of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Amora-Silva, B.F., Ribeiro, S.C., Vieira, C.L. et al. Clinical efficacy of new α-bisabolol mouthwashes in postoperative complications of maxillofacial surgeries: a randomized, controlled, triple-blind clinical trial. Clin Oral Invest 23, 577–584 (2019). https://doi.org/10.1007/s00784-018-2464-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2464-4