Abstract
Objectives
Tooth-colored composites have emerged as a standard restorative material in caries therapy and have largely replaced materials such as silver amalgam or glass ionomer cements. In addition to their superior esthetics and desirable mechanical properties, composites also comprise negative characteristics, such as wear, shrinkage, and an adverse biocompatibility. Modifications of classic resin-based dental composites have been developed to overcome these shortcomings. For example, ormocers are innovative inorganic-organic hybrid polymers that form a siloxane network modified by the incorporation of organic groups. Recently, a new ormocer, Admira Fusion (VOCO), was introduced to composite technology. The absence of cytotoxic matrix monomers leads to the hypothesis that ormocers have improved biocompatibility compared to resin-based dental restorative materials.
Materials and methods
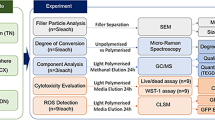
The aim of this study was to compare the cytotoxic effects of Admira Fusion to a nanohybrid composite (GrandioSO, VOCO) and a nanofiller composite (Filtek Supreme XTE, 3M Espe) on the standard dermal mouse fibroblasts (L929) and human gingival fibroblasts (GF-1) via a Cell Counting Kit-8 (CCK-8) assay.
Results
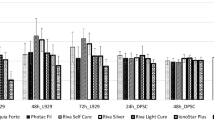
Admira Fusion was significantly less cytotoxic than GrandioSO and Filtek Supreme XTE to both the standard mouse dermal fibroblasts (L929) and human gingival fibroblasts.
Conclusions
Compared to other resin-based dental restorative materials, the ormocer (Admira Fusion) possesses a superior biocompatibility in vitro. Future research studies are needed to confirm our results.
Clinical significance
Clinically, dental practitioners and their patients might benefit from Admira Fusion in terms of reduced adverse biologic reactions compared to resin-based dental restorative materials.



Similar content being viewed by others
References
Goldberg M (2008) In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig 12(1):1–8
Ferracane JL (1994) Elution of leachable components from composites. J Oral Rehabil 21(4):441–452
Carmichael AJ, Gibson JJ, Walls AW (1997) Allergic contact dermatitis to bisphenol-A-glycidyldimethacrylate (BIS-GMA) dental resin associated with sensitivity to epoxy resin. Br Dent J 183(8):297–298
Geurtsen W, Lehmann F, Spahl W, Leyhausen G (1998) Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res 41(3):474–480
Engelmann J, Leyhausen G, Leibfritz D, Geurtsen W (2002) Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J Biomed Mater Res 63(6):746–751
Chang HH, Guo MK, Kasten FH, Chang MC, Huang GF, Wang YL, Wang RS, Jeng JH (2005) Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 26(7):745–753
Stanislawski L, Daniau X, Lauti A, Goldberg M (1999) Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J Biomed Mater Res 48(3):277–288
Jaffer F, Finer Y, Santerre JP (2002) Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials 23(7):1707–1719
Fleming GJ, Hall DP, Shortall AC, Burke FJ (2005) Cuspal movement and microleakage in premolar teeth restored with posterior filling materials of varying reported volumetric shrinkage values. J Dent 33(2):139–146
Lutz F, Krejci I (2000) Amalgam substitutes: a critical analysis. J Esthet Dent 12(3):146–159
Gregor L, Dorien L, Bortolotto T, Feilzer AJ, Krejci I (2016) Marginal integrity of low-shrinking versus methacrylate-based composite: effect of different one-step self-etch adhesives. Odontology
Susila AV, Balasubramanian V (2016) Correlation of elution and sensitivity of cell lines to dental composites. Dent Mater 32(3):e63–e72
Al-Ahdal K, Silikas N, Watts DC (2015) Development of viscoelastic stability of resin-composites incorporating novel matrices. Dent Mater 31(12):1561–1566
Bacchi A, Feitosa VP, da Silva Fonseca AS, Cavalcante LM, Silikas N, Schneider LF (2015) Shrinkage, stress, and modulus of dimethacrylate, ormocer, and silorane composites. J Conserv Dent 18(5):384–388
Wolter H, Storch W, Ott H (1994) New inorganic/organic copolymers (ORMOCERs) for dental applications. Mater Res Soc Symp Proc 346:143–149
Mahmoud SH, El-Embaby AE, AbdAllah AM (2014) Clinical performance of ormocer, nanofilled, and nanoceramic resin composites in class I and class II restorations: a three-year evaluation. Oper Dent 39(1):32–42
Monsarrat P, Garnier S, Vergnes JN, Nasr K, Grosgogeat B, Joniot S (2017) Survival of directly placed ormocer-based restorative materials: a systematic review and meta-analysis of clinical trials. Dent Mater 33(5):e212-e20
Moszner N, Gianasmidis A, Klapdohr S, Fischer UK, Rheinberger V (2008) Sol-gel materials 2. Light-curing dental composites based on ormocers of cross-linking alkoxysilane methacrylates and further nano-components. Dent Mater 24(6):851–856
Al-Hiyasat AS, Darmani H, Milhem MM (2005) Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig 9(1):21–25
Kydd WL, Daly CH (1982) The biologic and mechanical effects of stress on oral mucosa. J Prosthet Dent 47(3):317–329
Skin H-PA (1998) Mucosal reactions associated with dental materials. Eur J Oral Sci 106(2 Pt 2):707–712
Wataha JC, Lockwood PE, Bouillaguet S, Noda M (2003) In vitro biological response to core and flowable dental restorative materials. Dent Mater 19(1):25–31
Hensten-Pettersen A, Helgeland K (1981) Sensitivity of different human cell lines in the biologic evaluation of dental resin-based restorative materials. Scand J Dent Res 89(1):102–107
Theilig C, Tegtmeier Y, Leyhausen G, Geurtsen W (2000) Effects of BisGMA and TEGDMA on proliferation, migration, and tenascin expression of human fibroblasts and keratinocytes. J Biomed Mater Res 53(6):632–639
Eljezi T, Pinta P, Richard D, Pinguet J, Chezal JM, Chagnon MC, Sautou V, Grimandi G, Moreau E (2017) In vitro cytotoxic effects of DEHP-alternative plasticizers and their primary metabolites on a L929 cell line. Chemosphere 173:452–459
Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K (1997) A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44(7):1299–1305
He SJ, Cao J, Li YS, Yang JC, Zhou M, Qu CY, Zhang Y, Shen F, Chen Y, Li MM, Xu LM (2016) CdSe/ZnS quantum dots induce photodynamic effects and cytotoxicity in pancreatic cancer cells. World J Gastroenterol 22(21):5012–5022
Liu S, Yang H, Wan L, Cai HW, Li SF, Li YP, Cheng JQ, Lu XF (2011) Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol Sin 32(1):79–88
Enzenhofer E, Kadletz L, Stanisz I, Kotowski U, Seemann R, Schmid R, Thurnher D, Heiduschka G (2017) Effect of the histone deacetylase inhibitor resminostat on head and neck squamous cell carcinoma cell lines. Head Neck 39:900–907
Miyamoto T, Min W, Lillehoj HS (2002) Lymphocyte proliferation response during Eimeria tenella infection assessed by a new, reliable, nonradioactive colorimetric assay. Avian Dis 46(1):10–16
Failli A, Legitimo A, Orsini G, Castagna M, Spisni R, Miccoli P, Consolini R (2013) Antiproliferative effects of 5-fluorouracil and oxaliplatin in colon cancer cell lines: comparison of three different cytotoxicity assays. J Biol Regul Homeost Agents 27(1):275–284
Durner J, Obermaier J, Draenert M, Ilie N (2012) Correlation of the degree of conversion with the amount of elutable substances in nano-hybrid dental composites. Dent Mater 28(11):1146–1153
Hanks CT, Strawn SE, Wataha JC, Craig RG (1991) Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res 70(11):1450–1455
Batarseh G, Windsor LJ, Labban NY, Liu Y, Gregson K (2014) Triethylene glycol dimethacrylate induction of apoptotic proteins in pulp fibroblasts. Oper Dent 39(1):E1–E8
Chang HH, Chang MC, Lin LD, Lee JJ, Wang TM, Huang CH, Yang TT, Lin HJ, Jeng JH (2010) The mechanisms of cytotoxicity of urethane dimethacrylate to Chinese hamster ovary cells. Biomaterials 31(27):6917–6925
Alshali RZ, Salim NA, Sung R, Satterthwaite JD, Silikas N (2015) Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent Mater 31(12):1587–1598
Amato PA, Martins RP, dos Santos Cruz CA, Capella MV, Martins LP (2014) Time reduction of light curing: influence on conversion degree and microhardness of orthodontic composites. Am J Orthod Dentofac Orthop 146(1):40–46
Caughman WF, Caughman GB, Shiflett RA, Rueggeberg F, Schuster GS (1991) Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials 12(8):737–740
Randolph LD, Palin WM, Leloup G, Leprince JG (2016) Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent Mater 32(12):1586–1599
Bouillaguet S, Shaw L, Gonzalez L, Wataha JC, Krejci I (2002) Long-term cytotoxicity of resin-based dental restorative materials. J Oral Rehabil 29(1):7–13
Ferracane JL, Condon JR (1990) Rate of elution of leachable components from composite. Dent Mater 6(4):282–287
Moharamzadeh K, Van Noort R, Brook IM, Scutt AM (2007) Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater 23(1):40–44
Acknowledgements
We would like to thank VOCO for the financial support.
Funding
This work was supported by VOCO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
We dedicate this work to our lab technician Andrea Bernhard who passed away shortly after the results were gathered.
Rights and permissions
About this article
Cite this article
Schubert, A., Ziegler, C., Bernhard, A. et al. Cytotoxic effects to mouse and human gingival fibroblasts of a nanohybrid ormocer versus dimethacrylate-based composites. Clin Oral Invest 23, 133–139 (2019). https://doi.org/10.1007/s00784-018-2419-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2419-9