Abstract
Objectives
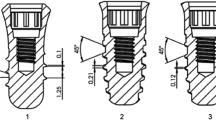
The objective of this study was to assess, by histomorphometric analysis, the degree of bone apposition on two types of dental implant’s surfaces: a novel implant that combines Al2O3 abrasive particle blasting with thermochemical treatment (ContacTi), compared to a standard surface treatment obtained by sandblasting and acid etching (shot blasting).
Materials and methods
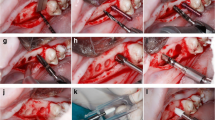
Twelve minipigs were used, placing the studied implants in the maxillae, and divided into three groups according to the time of sacrifice: 2, 4, and 8 weeks after implant placement. Histological and histomorphometric analyses were performed following standardized tissue polymerization, cutting, and staining and examined under optical and high-resolution electron microscope.
Results
For all measurements, the novel surface presented higher levels of osseointegration as compared to the shot blasting surface. Bone to implant contact (BIC) in the maxillae for ContacTi presented values of 49.02, 83.20, and 85.58% at 2, 4, and 8 weeks, respectively, significantly higher compared to the shot blasting surface values of 39.32, 46.53, and 46.20% for the same time points. Bone area density (BAD) presented values of 26.52, 61.21, and 59.50% for ContacTi surface implants and 22.95, 36.26, and 49.50% for the shot blasted surface implants. Signs of osteoconductivity were observed in the ContacTi surfaces at 2 weeks.
Conclusions
The ContacTi surface achieved a faster growth of hard tissues around the implants, when compared to the shot blasting surface, and for all evaluated histomorphometric parameters, the values were higher at all measured time points.
Clinical relevance
ContacTi could be a new surface improving the osseointegration in oral implantology.









Similar content being viewed by others
References
Steigenga JT, al-Shammari KF, Nociti FH et al (2003) Dental implant design and its relationship to long-term implant success. Implant Dent 12:306–317. https://doi.org/10.1097/01.ID.0000091140.76130.A1
Aljateeli M, Wang HL (2013) Implant microdesigns and their impact on osseointegration. Implant Dent 22:127–132. https://doi.org/10.1097/ID.0b013e318278a90b
Albrektsson T, Wennerberg A Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 17:536–43
Albrektsson T, Wennerberg A (2004) Oral implant surfaces: Part 2--review focusing on clinical knowledge of different surfaces. Int J Prosthodont 17:544–564. https://doi.org/10.1098/rsta.2009.0062
Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF (2000) Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 22:87–96. https://doi.org/10.1016/S0142-9612(00)00174-5
Lamers E, Frank Walboomers X, Domanski M et al (2010) The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 31:3307–3316. https://doi.org/10.1016/j.biomaterials.2010.01.034
Lincks J, Boyan BD, Blanchard CR et al (2006) Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomater Silver Jubil Compend 19:147–160. https://doi.org/10.1016/B978-008045154-1.50019-8
Von Der Mark K, Park J, Bauer S, Schmuki P (2010) Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res 339:131–153. https://doi.org/10.1007/s00441-009-0896-5
Aparicio C, Gil FJ, Fonseca C et al (2003) Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 24:263–273
Gil FJ, Planell JA, Padrós A (2002) Fracture and fatigue behavior of shot-blasted titanium dental implants. Implant Dent 11:28–32
Stanford CM (2008) Surface modifications of dental implants. Aust Dent J. https://doi.org/10.1111/j.1834-7819.2008.00038.x
Bressan E, Sbricoli L, Guazzo R et al (2013) Nanostructured surfaces of dental implants. Int J Mol Sci 14:1918–1931. https://doi.org/10.3390/ijms14011918
Novaes AB, de Souza SLS, de Barros RRM et al (2010) Influence of implant surfaces on osseointegration. Braz Dent J 21:471–481. https://doi.org/10.1590/S0103-64402010000600001
Mendes VC, Moineddin R, Davies JE (2009) Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J Biomed Mater Res - Part A 90:577–585. https://doi.org/10.1002/jbm.a.32126
Tanaka Y, Matin K, Gyo M et al (2010) Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J Biomed Mater Res - Part A 95:1105–1113. https://doi.org/10.1002/jbm.a.32932
Kokubo T, Miyaji F, Kim H-M, Nakamura T (1996) Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J Am Ceram Soc 79:1127–1129. https://doi.org/10.1111/j.1151-2916.1996.tb08561.x
Yan WQ, Nakamura T, Kawanabe K et al (1997) Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 18:1185–1190. https://doi.org/10.1016/S0142-9612(97)00057-4
Yan WQ, Nakamura T, Kobayashi M et al (1997) Bonding of chemically treated titanium implants to bone. J Biomed Mater Res 37:267–275. https://doi.org/10.1002/(SICI)1097-4636(199711)37:2<267::AID-JBM17>3.0.CO;2-B
Aparicio C, Padrós A, Gil F-J (2011) In vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J Mech Behav Biomed Mater 4:1672–1682. https://doi.org/10.1016/j.jmbbm.2011.05.005
Aparicio C, Manero JM, Conde F et al (2007) Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J Biomed Mater Res - Part A 82:521–529. https://doi.org/10.1002/jbm.a.31164
Gil F, Padrós A, Manero J et al (2002) Growth of bioactive surfaces on titanium and its alloys for orthopaedic and dental implants. Mater Sci Eng C 22:53–60. https://doi.org/10.1016/S0928-4931(01)00389-7
Buser D, Nydegger T, Oxland T et al (1999) Interface shear strength of titanium implants with a sandblasted and acid-etched surface: a biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res 45:75–83
Buser D, Broggini N, Wieland M et al (2004) Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 83:529–533. https://doi.org/10.1177/154405910408300704
Germanier Y, Tosatti S, Broggini N et al (2006) Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in miniature pigs. Clin Oral Implants Res 17:251–257. https://doi.org/10.1111/j.1600-0501.2005.01222.x
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326
Puleo D, Nanci A (1999) Understanding and controlling the bone–implant interface. Biomaterials 20:2311–2321. https://doi.org/10.1016/S0142-9612(99)00160-X
Rønold HJ, Lyngstadaas SP, Ellingsen JE (2003) Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials 24:4559–4564. https://doi.org/10.1016/S0142-9612(03)00256-4
Geesink RGT, De Groot K, Klein CPAT (1987) Chemical Implant Fixation Using Hydroxyl-Apatite Coatings. Clin Orthop Relat Res:147–170
Shirkhanzadeh M (1991) Bioactive calcium phosphate coatings prepared by electrodeposition. J Mater Sci Lett 10:1415–1417. https://doi.org/10.1007/BF00735695
Hulshoff JEG, Hayakawa T, Van Dijk K et al (1997) Mechanical and histologic evaluation of Ca-P plasma-spray and magnetron sputter-coated implants in trabecular bone of the goat. J Biomed Mater Res 36:75–83. https://doi.org/10.1002/(SICI)1097-4636(199707)36:1<75::AID-JBM9>3.0.CO;2-I
Favero R, Botticelli D, Antunes AA et al (2016) Sequential Healing at Calcium- versus Calcium Phosphate-Modified Titanium Implant Surfaces: An Experimental Study in Dogs. Clin Implant Dent Relat Res 18:369–378. https://doi.org/10.1111/cid.12311
Favero V, Lang NP, Favero R et al (2016) Sequential morphometric evaluation at UnicCa(®) and DCD(®) implant surfaces. An experimental study in the dog. Clin Oral Implants Res. https://doi.org/10.1111/clr.12888
Botticelli D, Lang NP (2016) Dynamics of osseointegration in various human and animal models - a comparative analysis. Clin Oral Implants Res. https://doi.org/10.1111/clr.12872
Gahlert M, Roehling S, Sprecher CM et al (2012) In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clin Oral Implants Res 23:281–286. https://doi.org/10.1111/j.1600-0501.2011.02157.x
Schwarz F, Herten M, Sager M et al (2007) Bone regeneration in dehiscence-type defects at chemically modified (SLActive®) and conventional SLA titanium implants: A pilot study in dogs. J Clin Periodontol 34:78–86. https://doi.org/10.1111/j.1600-051X.2006.01008.x
Lang NP, Salvi GE, Huynh-Ba G et al (2011) Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res 22:349–356. https://doi.org/10.1111/j.1600-0501.2011.02172.x
Bosshardt DD, Salvi GE, Huynh-Ba G et al (2011) The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin Oral Implants Res 22:357–364. https://doi.org/10.1111/j.1600-0501.2010.02107.x
Favero R, Lang NP, Salata LA et al (2016) Sequential healing events of osseointegration at UnicCa(®) and SLActive(®) implant surfaces: an experimental study in the dog. Clin Oral Implants Res 27:203–210. https://doi.org/10.1111/clr.12591
Rossi F, Lang NP, De Santis E et al (2014) Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin Oral Implants Res 25:124–131. https://doi.org/10.1111/clr.12097
Gil FJ, Manzanares N, Badet A et al (2014) Biomimetic treatment on dental implants for short-term bone regeneration. Clin Oral Investig 18:59–66. https://doi.org/10.1007/s00784-013-0953-z
Groessner-Schreiber B, Tuan RS (1992) Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J Cell Sci 101 ( Pt 1:209–217
Fischer K, Stenberg T (2004) Early loading of ITI implants supporting a maxillary full-arch prosthesis: 1-year data of a prospective, randomized study. Int J Oral Maxillofac Implants 19:374–381
Gottlow J, Dard M, Kjellson F et al (2012) Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin Implant Dent Relat Res 14:538–545. https://doi.org/10.1111/j.1708-8208.2010.00289.x
Acknowledgments
The authors would like to acknowledge the Ministry of Science of Spain for funding to this project (MAT2012-30706) as well as Klockner, S.L. for donating the implants used.
Funding
The work was supported by the Spanish government. Ministerio Economía y Competitividad by the research project number MAT2012-30706.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was carried out in maxillae of 12 six-year-old female minipigs in the Córdoba University’s Servicio Centralizado de Animales de Experimentación located in the Campus de Rabanales and approved by the University of Seville Ethics Experimentation Committee (MED2016-01-324). All requirements and regulations for animal experimentation, according to the Spanish and European Union, were fulfilled.
Conflict of interest
Mariano Herrero-Climent declares that he has no conflict of interest. Manuel María Romero declares that he has no conflict of interest. Pedro Lázaro declares that he has no conflict of interest. José Vicente Rios declares that he has no conflict of interest, and F. Javier Gil Mur declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. For the animal study, the study was approved by the University of Seville Ethics Experimentation Committee (MED2016-01-324).
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Herrero-Climent, M., Romero Ruizª, M.M., Calvo, P.L. et al. Effectiveness of a new dental implant bioactive surface: histological and histomorphometric comparative study in minipigs. Clin Oral Invest 22, 1423–1432 (2018). https://doi.org/10.1007/s00784-017-2223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2223-y