Abstract
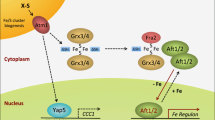
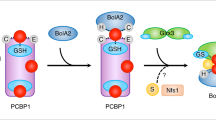
The Saccharomyces cerevisiae transcriptional activator Aft1 and its paralog Aft2 respond to iron deficiency by upregulating expression of proteins required for iron uptake at the plasma membrane, vacuolar iron transport, and mitochondrial iron metabolism, with the net result of mobilizing iron from extracellular sources and intracellular stores. Conversely, when iron levels are sufficient, Aft1 and Aft2 interact with the cytosolic glutaredoxins Grx3 and Grx4 and the BolA protein Bol2, which promote Aft1/2 dissociation from DNA and subsequent export from the nucleus. Previous studies unveiled the molecular mechanism for iron-dependent inhibition of Aft1/2 activity, demonstrating that the [2Fe–2S]-bridged Grx3–Bol2 heterodimer transfers a cluster to Aft2, driving Aft2 dimerization and dissociation from DNA. Here, we provide further insight into the regulation mechanism by investigating the roles of conserved cysteines in Aft2 in iron–sulfur cluster binding and interaction with [2Fe–2S]–Grx3–Bol2. Using size exclusion chromatography and circular dichroism spectroscopy, these studies reveal that both cysteines in the conserved Aft2 Cys-Asp-Cys motif are essential for Aft2 dimerization via [2Fe–2S] cluster binding, while only one cysteine is required for interaction with the [2Fe–2S]–Grx3–Bol2 complex. Taken together, these results provide novel insight into the molecular details of iron–sulfur cluster transfer from Grx3–Bol2 to Aft2 which likely occurs through a ligand exchange mechanism. Loss of either cysteine in the Aft2 iron–sulfur binding site may disrupt this ligand-exchange process leading to the isolation of a trapped Aft2–Grx3–Bol2 intermediate, while the replacement of both cysteines abrogates both the iron–sulfur cluster exchange and the protein–protein interactions between Aft2 and Grx3–Bol2.





Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- CDC:
-
Cys–Asp–Cys
- Cys:
-
Cysteine
- Fe–S:
-
Iron–sulfur
- Grx:
-
Glutaredoxin
- GSH:
-
Glutathione
- IPTG:
-
Isopropyl β-d-thiogalactoside
- LB:
-
Luria–Bertani medium
- PMSF:
-
Phenylmethanesulfonyl fluoride
- SEC:
-
Size exclusion chromatography
- TCEP:
-
Tris[2-carboxyethyl] phosphine
- WT:
-
Wild type
References
Blaiseau PL, Lesuisse E, Camadro JM (2001) J Biol Chem 276:34221–34226
Rutherford JC, Jaron S, Ray E, Brown PO, Winge DR (2001) Proc Natl Acad Sci U S A 98:14322–14327
Yamaguchi-Iwai Y, Dancis A, Klausner RD (1995) EMBO J 14:1231–1239
Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD (1996) EMBO J 15:3377–3384
Courel M, Lallet S, Camadro JM, Blaiseau PL (2005) Mol Cell Biol 25:6760–6771
Rutherford JC, Jaron S, Winge DR (2003) J Biol Chem 278:27636–27643
Yamaguchi-Iwai Y, Ueta R, Fukunaka A, Sasaki R (2002) J Biol Chem 277:18914–18918
Rutherford JC, Ojeda L, Balk J, Mühlenhoff U, Lill R, Winge DR (2005) J Biol Chem 280:10135–10140
Kumanovics A, Chen OS, Li L, Bagley D, Adkins EM, Lin H, Dingra NN, Outten CE, Keller G, Winge D, Ward DM, Kaplan J (2008) J Biol Chem 283:10276–10286
Lesuisse E, Knight SA, Courel M, Santos R, Camadro JM, Dancis A (2005) Genetics 169:107–122
Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA (2006) J Cell Sci 119:4554–4564
Mühlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, Lillig CH, Lill R (2010) Cell Metab 12:373–385
Ueta R, Fujiwara N, Iwai K, Yamaguchi-Iwai Y (2012) Mol Cell Biol 32:4998–5008
Poor CB, Wegner SV, Li H, Dlouhy AC, Schuermann JP, Sanishvili R, Hinshaw JR, Riggs-Gelasco PJ, Outten CE, He C (2014) Proc Natl Acad Sci USA 111:4043–4048
Li H, Mapolelo DT, Dingra NN, Keller G, Riggs-Gelasco PJ, Winge DR, Johnson MK, Outten CE (2011) J Biol Chem 286:867–876
Li H, Mapolelo DT, Dingra NN, Naik SG, Lees NS, Hoffman BM, Riggs-Gelasco PJ, Huynh BH, Johnson MK, Outten CE (2009) Biochemistry 48:9569–9581
Li H, Outten CE (2012) Biochemistry 51:4377–4389
Picciocchi A, Saguez C, Boussac A, Cassier-Chauvat C, Chauvat F (2007) Biochemistry 46:15018–15026
Ojeda L, Keller G, Mühlenhoff U, Rutherford JC, Lill R, Winge DR (2006) J Biol Chem 281:17661–17669
Ueta R, Fujiwara N, Iwai K, Yamaguchi-Iwai Y (2007) Mol Biol Cell 18:2980–2990
Acknowledgements
This work was supported by Grant R35 GM118164 to C.E.O. from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Outten, C.E. The conserved CDC motif in the yeast iron regulator Aft2 mediates iron–sulfur cluster exchange and protein–protein interactions with Grx3 and Bol2. J Biol Inorg Chem 24, 809–815 (2019). https://doi.org/10.1007/s00775-019-01705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01705-x