Abstract
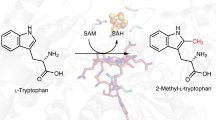
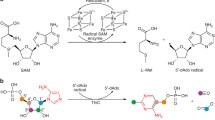
The cobalamin-dependent radical S-adenosylmethionine (SAM) enzyme TsrM catalyzes the methylation of C2 of l-tryptophan to form 2-methyltryptophan during the biosynthesis of thiostrepton A. Although TsrM is a member of the radical SAM superfamily, unlike all other annotated members, it does not catalyze a reductive cleavage of SAM to a 5′-deoxyadenosyl 5′-radical intermediate. In fact, it has been proposed that TsrM catalyzes its reaction through two polar nucleophilic displacements, with its cobalamin cofactor cycling directly between methylcobalamin (MeCbl) and cob(I)alamin. Nevertheless, the enzyme has been stated to require the action of a reductant, which can be satisfied by dithiothreitol. By contrast, all other annotated RS enzymes require a reductant that exhibits a much lower reduction potential, which is necessary for the reductive cleavage of SAM. Herein, we show that TsrM can catalyze multiple turnovers in the absence of any reducing agent, but only when it is pre-loaded with MeCbl. When hydroxocobalamin (OHCbl) or cob(II)alamin is bound to TsrM, a reductant is required to convert it to cob(I)alamin, which can acquire a methyl group directly from SAM. Our studies suggest that TsrM uses an external reductant to prime its reaction by converting bound OHCbl or cob(II)alamin to MeCbl, and to regenerate the MeCbl form of the cofactor upon adventitious oxidation of the cob(I)alamin intermediate to cob(II)alamin.







Similar content being viewed by others
Abbreviations
- 5′-dA:
-
5′-deoxyadenosyl radical
- β:
-
Mercaptoethanol
- DTT:
-
Dithiothreitol
- flr:
-
Flavodoxin reductase
- flv:
-
Flavodoxin
- LC–MS/MS:
-
Liquid chromatography tandem mass spectrometry
- MeCbl:
-
Methylcobalamin
- MetH:
-
Methionine synthase
- MeTrp:
-
2-methyltryptophan
- RS:
-
Radical S-adenosylmethionine
- SAM:
-
S-adenosylmethionine
- TCEP:
-
Tris (2-carboxyethyl) phosphine
- Trp:
-
Tryptophan
References
Li C, Kelly WL (2010) Recent advances in thiopeptide antibiotic biosynthesis. Nat Prod Rep 27:153–164
Pierre S, Guillot A, Benjdia A, Sandström C, Langella P, Berteau O (2012) Thiostrepton tryptophan methyltransferase expands the chemistry of radical SAM enzymes. Nat Chem Biol 8:957–959
Blaszczyk AJ, Silakov A, Zhang B, Maiocco SJ, Lanz ND, Kelly WL, Elliott SJ, Krebs C, Booker SJ (2016) Spectroscopic and electrochemical characterization of the iron-sulfur and cobalamin cofactors of TsrM, an unusual radical S-adenosylmethionine methylase. J Am Chem Soc 138:3416–3426
Blaszczyk AJ, Wang B, Silakov A, Ho JV, Booker SJ (2017) Efficient methylation of C2 in l-tryptophan by the cobalamin-dependent radical S-adenosylmethionine methylase TsrM requires an unmodified N1 amine. J Biol Chem 292:15456–15467
Banerjee RV, Matthews RG (1990) Cobalamin-dependent methionine synthase. FASEB J 4:1450–1459
Drummond JT, Huang S, Blumenthal RM, Matthews RG (1993) Assignment of enzymatic function to specific protein regions of cobalamin-dependent methionine synthase from Escherichia coli. Biochemistry 32:9290–9295
Fujii K, Galivan JH, Huennekens FM (1977) Activation of methionine synthase: further characterization of flavoprotein system. Arch Biochem Biophys 178:662–670
Arcinas AJ, Maiocco SJ, Elliott SJ, Silakov A, Booker SJ (2019) Ferredoxins as interchangeable redox components in support of MiaB, a radical S-adenosylmethionine methylthiotransferase. Protein Sci 28:267–282
Maiocco SJ, Arcinas AJ, Booker SJ, Elliott SJ (2019) Parsing redox potentials of five ferredoxins found within Thermotoga maritima. Protein Sci 28:257–266
Iwig DF, Booker SJ (2004) Insight into the polar reactivity of the onium chalcogen analogues of S-adenosyl-l-methionine. Biochemistry 43:13496–13509
Cicchillo RM, Iwig DF, Jones AD, Nesbitt NM, Baleanu-Gogonea C, Souder MG, Tu L, Booker SJ (2004) Lipoyl synthase requires two equivalents of S-adenosyl-l-methionine to synthesize one equivalent of lipoic acid. Biochemistry 43:6378–6386
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lanz N, Blaszczyk AJ, McCarthy E, Wang B, Wang R, Jones B, Booker SJ (2018) Enhanced solubilization of class B radical S-adenosylmethionine methylases by improved cobalamin uptake in Escherichia coli. Biochemistry 57(9):1475–1490
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ljungdahl LG, LeGall J, Lee JP (1973) Isolation of a protein containing tightly bound 5-methoxybenzimidazolylcobamide (factor 3m) from Clostridium thermoaceticum. Biochemistry 12:1802–1808
Blaszczyk AJ, Wang RX, Booker SJ (2017) TsrM as a model for purifying and characterizing cobalamin-dependent radical S-adenosylmethionine methylases. Methods Enzymol 595:303–329
Jenkins CM, Waterman MR (1998) NADPH-flavodoxin reductase and flavodoxin from Escherichia coli: characteristics as a soluble microsomal P450 reductase. Biochemistry 37:6106–6113
Hoover DM, Jarrett JT, Sands RH, Dunham WR, Ludwig ML, Matthews RG (1997) Interaction of Escherichia coli cobalamin-dependent methionine synthase and its physiological partner flavodoxin: binding of flavodoxin leads to axial ligand dissociation from the cobalamin cofactor. Biochemistry 36:127–138
Pullela PK, Chiku T, Carvan MJ III, Sem DS (2006) Fluorescence-based detection of thiols in vitro and in vivo using dithiol probes. Anal Biochem 352:265–273
Mickey B, Howard J (1995) Rigidity of microtubules is increased by stabilizing agents. J Cell Biol 130:909–917
Cleland WW (1964) Dithiothretol, a new protective reagent for SH groups. Biochemistry 3:480–482
Creutz C (1981) Complexities of ascorbate as a reducing agent. Inorg Chem 20:4449–4452
Otvos JD, Krum DP, Masters BS (1986) Localization of the free radical on the flavin mononucleotide of the air-stable semiquinone state of NADPH-cytochrome P-450 reductase using 31P NMR spectroscopy. Biochemistry 25:7220–7228
Jenkins CM, Waterman MR (1994) Flavodoxin and NADPH-flavodoxin reductase from Escherichia coli support bovine cytochrome P450c17 hydroxylase activities. J Biol Chem 269:27401–27408
Mayhew SG (1978) The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem 85:535–547
Mayhew SG, Foust GP, Massey V (1969) Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem 244:803–810
Jarrett JT, Goulding CW, Fluhr K, Huang S, Matthews RG (1997) Purification and assay of cobalamin-dependent methionine synthase from Escherichia coli. Methods Enzymol 281:196–213
Menon S, Ragsdale SW (1998) Role of the [4Fe-4S] cluster in reductive activation of the cobalt center of the corrinoid iron–sulfur protein from Clostridium thermoaceticum during acetate biosynthesis. Biochemistry 37:5689–5698
Menon S, Ragsdale SW (1999) The role of an iron-sulfur cluster in an enzymatic methylation reaction. Methylation of CO dehydrogenase/acetyl-CoA synthase by the methylated corrinoid iron-sulfur protein. J Biol Chem 274:11513–11518
Salnikov DS, Silaghi-Dumitrescu R, Makarov SV, van Eldik R, Boss GR (2011) Cobalamin reduction by dithionite. Evidence for the formation of a six-coordinate cobalamin(II) complex. Dalton Trans 40:9831–9834
Funding
This work was supported an NIH grant (GM-122595) and the Eberly Family Distinguished Chair in Science to S. J. B, who is also an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blaszczyk, A.J., Knox, H.L. & Booker, S.J. Understanding the role of electron donors in the reaction catalyzed by Tsrm, a cobalamin-dependent radical S-adenosylmethionine methylase. J Biol Inorg Chem 24, 831–839 (2019). https://doi.org/10.1007/s00775-019-01689-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01689-8