Abstract
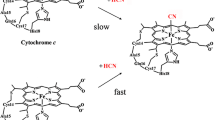
Microperoxidase-11 (MP11) is an undecapeptide derived from horse heart cytochrome c, which is considered as a heme-protein model. Here, the reductive nitrosylation of ferric MP11 (MP11(III)) under anaerobic conditions has been investigated between pH 7.4 and 9.2, at T = 20.0 °C. At pH ≤ 7.7, NO binds reversibly to MP11(III) leading to the formation of the MP11(III)–NO complex. However, between pH 8.2 and 9.2, the addition of NO to MP11(III) leads to the formation of ferrous nitrosylated MP11(II) (MP11(II)–NO). In fact, the transient MP11{FeNO}6 species is converted to ferrous deoxygenated MP11 (MP11(II)) by OH−- and H2O-based catalysis, which represents the rate-limiting step of the whole reaction. Then, MP11(II) binds NO very rapidly leading to MP11(II)–NO formation. Over the whole pH range explored, the apparent values of kon, koff, and K (= koff/kon) for MP11(III)(–NO) (de)nitrosylation are essentially pH independent, ranging between 5.8 × 105 M−1 s−1 and 1.6 × 106 M−1 s−1, between 1.9 s−1 and 3.7 s−1, and between 1.4 × 10−6 M and 4.6 × 10−6 M, respectively. Values of the apparent pseudo-first-order rate constant for the MP11{FeNO}6 conversion to MP11(II) (i.e., h) increase linearly with pH; the apparent values \(h_{\text{OH}^{-}}\) and \(h_{{{\text{H}}_{ 2} {\text{O}}}}\) are 7.2 × 102 M−1 s−1 and 2.5 × 10−4 s−1, respectively. Present data confirm that MP11 is a useful molecular model to highlight the role of the protein matrix on the heme-based reactivity.




Similar content being viewed by others
Notes
The term “penta-coordinated ferric heme-proteins” is usually used from biochemists to indicate metal centers either non-coordinating or eventually coordinating a water molecule whose dissociation rate never limits the binding of exogenous ligands (e.g., azide). As a case, the dissociation rate constant of the water molecule from ferric sperm whale Mb is 3.8 × 102 s−1 [47].
Abbreviations
- MP11:
-
Microperoxidase-11
- MP11(III):
-
Ferric MP11
- MP11(III)-NO:
-
Nitrosylated MP11(III)
- MP11(II):
-
Ferrous MP11
- MP11(II)-NO:
-
Nitrosylated MP11(II)
- cytc :
-
Cytochrome c
- CL–cytc :
-
Cardiolipin-bound cytc
- CM–cytc :
-
Carboxymethylated cytc
- Mb:
-
Myoglobin
- Ngb:
-
Neuroglobin
- SA:
-
Serum albumin
- HPX:
-
Hemopexin
References
Harbury HA, Loach PA (1960) J Biol Chem 235:3640–3645
Wilson MT, Ranson RJ, Masiakowski P, Czarnecka E, Brunori M (1977) Eur J Biochem 77:193–199
Aron J, Baldwin DA, Marques HM, Pratt JM, Adams PA (1986) J Inorg Biochem 27:227–243
Braun M, Thöny-Meyer L (2004) Proc Natl Acad Soc USA 101:12830–12835
Kleingardner EC, Asher WB, Bren KL (2017) Biochemistry 56:143–148
Ow Y-LP, Green, Hao Z, Mak TW (2008) Nat Rev Mol Cell Biol 9:532–542
Stevens JM (2011) Metallomics 3:319–322
Marques HM (2007) Dalton Trans 39:4371–4385
Sharma VS, Ranney HM, Geibel JF, Traylor TG (1975) Biochem Biophys Res Commun 66:1301–1306
Sharma VS, Isaacson RA, John ME, Waterman MR, Chevion M (1983) Biochemistry 22:3897–3902
Ascenzi P, Sbardella D, Santucci R, Coletta M (2016) J Biol Inorg Chem 21:511–522
Feder N (1971) J Cell Biol 51:339–343
Ascenzi P, Leboffe L, Santucci R, Coletta M (2015) J Inorg Biochem 144:56–61
Ascenzi P, Sbardella D, Fiocchetti M, Santucci R, Coletta M (2015) J Inorg Biochem 153:121–127
Reddy KS, Yonetani T, Tsuneshige A, Chance B, Kushkuley B, Stavrov SS, Vanderkooi JM (1996) Biochemistry 35:5562–5570
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their Reactions with ligands. North-Holland Publishing Co, Amsterdam
Ascenzi P, Brunori M, Pennesi G, Ercolani C, Monacelli F (1987) J Chem Soc Dalton Trans 2:369–371
Hoshino M, Maeda M, Konishi R, Seki H, Ford PC (1996) J Am Chem Soc 118:5702–5707
Boffi A, Sarti P, Amiconi G, Chiancone E (2002) Biophys Chem 98:209–216
Herold S, Fago A, Weber RE, Dewilde S, Moens L (2004) J Biol Chem 279:22841–22847
Herold S, Puppo A (2005) J Biol Inorg Chem 10:946–957
Ascenzi P, Bocedi A, Antonini G, Bolognesi M, Fasano M (2007) FEBS J 274:551–562
Ascenzi P, di Masi A, Gullotta F, Mattu M, Ciaccio C, Coletta M (2010) Biochem Biophys Res Commun 393:196–200
Ascenzi P, Yu C, di Masi A, Gullotta F, De Sanctis G, Fanali G, Fasano M, Coletta M (2010) FEBS J 277:2474–2485
Ascenzi P, Pesce A, Nardini M, Bolognesi M, Ciaccio C, Coletta M, Dewilde S (2013) Biochem Biophys Res Commun 430:1301–1305
Ascenzi P, Marino M, Ciaccio C, Santucci R, Coletta M (2014) IUBMB Life 66:438–447
Ascenzi P, di Masi A, Tundo GR, Pesce A, Visca P, Coletta M (2014) PLoS One 9:e102811
Ascenzi P, Bocedi A, Gioia M, Fanali G, Fasano M, Coletta M (2017) J Inorg Biochem 177:63–75
Ascenzi P, Ciaccio C, De Simone G, Santucci R, Coletta M (2017) J Porphyrins Phthalocyanines 21:1–9
Bateman H (1910) Proc Cambridge Phil Soc 15:423–427
Hill AV (1910) J Physiol 40:iv–vii
Hoshino M, Ozawa K, Seki H, Ford PC (1993) J Am Chem Soc 115:9568–9575
Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC (2003) J Biol Chem 278:51713–51721
Silkstone G, Kapetanaki SM, Husu I, Vos MH, Wilson MT (2012) Biochemistry 51:6760–6766
Andersen JF, Ding XD, Balfour C, Shokhireva TK, Champagne DE, Walker FA, Montfort WR (2000) Biochemistry 39:10118–10131
Montfort WR, Weichsel A, Andersen JF (2000) Biochim Biophys Acta 1482:110–118
Andersen JF (2010) Toxicon 56:1120–1129
Bianchetti CM, Blouin GC, Bitto E, Olson JS, Phillips GN Jr (2010) Proteins 78:917–931
De Simone G, Ascenzi P, di Masi A, Polticelli F (2017) Biomol Concepts 8:105–118
De Simone G, Ascenzi P, Polticelli F (2016) IUBMB Life 68:423–428
Fasano M, Bocedi A, Mattu M, Coletta M, Ascenzi P (2004) J Biol Inorg Chem 9:800–806
Moore EG, Gibson QH (1976) J Biol Chem 251:2788–2794
Silkstone G, Kapetanaki SM, Husu I, Vos MH, Wilson MT (2010) J Biol Chem 285:19785–19792
Perutz MF (1989) Trends Biochem Sci 14:42–44
Paoli M, Anderson BF, Baker HM, Morgan WT, Smith A, Baker EN (1999) Nat Struct Biol 6:926–931
Pettigrew WG, Moore RG (1987) Cytochromes c: Biological Aspects. Springer, Berlin
Coletta M, Angeletti M, De Sanctis G, Cerroni L, Giardina B, Amiconi G, Ascenzi P (1996) Eur J Biochem 235:49–53
Acknowledgements
Grant of Excellence Departments, MIUR (Legge 232/2016, Articolo 1, Comma 314-337), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ascenzi, P., De Simone, G., Sbardella, D. et al. Reductive nitrosylation of ferric microperoxidase-11. J Biol Inorg Chem 24, 21–29 (2019). https://doi.org/10.1007/s00775-018-1623-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-018-1623-z