Abstract
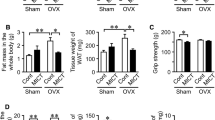
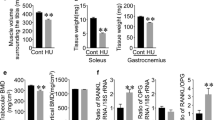
Androgen deficiency plays a crucial role in the pathogenesis of male osteoporosis and sarcopenia. Myokines have recently been identified as humoral factors that are involved in the interactions between muscle and bone; however, the influence of androgen deficiency on these interactions remains unclear. Therefore, we herein investigated the roles of humoral factors linking muscle to bone using orchidectomized mice with sarcopenia and osteopenia. Orchidectomy (ORX) significantly reduced muscle mass, grip strength, and trabecular bone mineral density (BMD) in mice. Among the myokines examined, ORX only significantly reduced fibronectin type III domain-containing 5 (Fndc5) mRNA levels in both the soleus and gastrocnemius muscles of mice. In simple regression analyses, Fndc5 mRNA levels in the soleus muscle positively correlated with trabecular BMD, but not cortical BMD. The administration of irisin, a product of Fndc5, significantly protected against the decrease induced in trabecular BMD, but not muscle mass, by androgen deficiency in mice. In conclusion, the present results demonstrated that androgen deficiency decreases the expression of irisin in the skeletal muscle of mice. Irisin may be involved in muscle/bone relationships negatively affected by androgen deficiency.







Similar content being viewed by others
References
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS (2012) Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:1802–1822
Khosla S (2010) Update in male osteoporosis. J Clin Endocrinol Metab 95:3–10
Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC (2017) Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97:135–187
Carson JA, Manolagas SC (2015) Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone 80:67–78
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS (2005) Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352:154–164
Mohamad NV, Soelaiman IN, Chin KY (2016) A concise review of testosterone and bone health. Clin Interv Aging 11:1317–1324
Russell PK, Clarke MV, Cheong K, Anderson PH, Morris HA, Wiren KM, Zajac JD, Davey RA (2015) Androgen receptor action in osteoblasts in male mice is dependent on their stage of maturation. J Bone Miner Res 30:809–823
Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, Vanderschueren D (2012) Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res 27:2535–2543
Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, Zou B, Borst SE, Yarrow JF (2018) Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 9:465–481
Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R (1997) Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82:407–413
Kaji H (2016) Effects of myokines on bone. Bonekey Rep 5:826
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695
Kawao N, Morita H, Obata K, Tatsumi K, Kaji H (2018) Role of follistatin in muscle and bone alterations induced by gravity change in mice. J Cell Physiol 233:1191–1201
Tanaka K, Matsumoto E, Higashimaki Y, Sugimoto T, Seino S, Kaji H (2012) FAM5C is a soluble osteoblast differentiation factor linking muscle to bone. Biochem Biophys Res Commun 418:134–139
Tanaka K, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, Kaji H (2012) Role of osteoglycin in the linkage between muscle and bone. J Biol Chem 287:11616–11628
Martinez Munoz IY, Camarillo Romero EDS, Garduno Garcia JJ (2018) Irisin a novel metabolic biomarker: present knowledge and future directions. Int J Endocrinol 2018:7816806
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, Hu Y, Xu W, Xu L (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep 6:18732
Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, Colucci S, Cinti S, Grano M (2014) Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014:902186
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 112:12157–12162
Kawao N, Moritake A, Tatsumi K, Kaji H (2018) Roles of irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif Tissue Int 103:24–34
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM (2018) Irisin mediates effects on bone and fat via αV integrin receptors. Cell 175:1756–1768 (e1717)
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G, Mori G, Brunetti G, Moretti B, Tarantino U, Colucci SC, Reseland JE, Vettor R, Cinti S, Grano M (2017) Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep 7:2811
Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, Jiang H, Dard MM, Huang J, Xu Z, Tu Q, Chen J (2017) Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res 5:16056
Tamura Y, Kawao N, Shimoide T, Okada K, Matsuo O, Kaji H (2018) Role of plasminogen activator inhibitor-1 in glucocorticoid-induced muscle change in mice. J Bone Miner Metab 36:148–156
Tamura Y, Fujito H, Kawao N, Kaji H (2017) Vitamin D deficiency aggravates diabetes-induced muscle wasting in female mice. Diabetol Int 8:52–58
Davis JS, Nastiuk KL, Krolewski JJ (2011) TNF is necessary for castration-induced prostate regression, whereas TRAIL and FasL are dispensable. Mol Endocrinol 25:611–620
Shiomi A, Kawao N, Yano M, Okada K, Tamura Y, Okumoto K, Matsuo O, Akagi M, Kaji H (2015) α2-Antiplasmin is involved in bone loss induced by ovariectomy in mice. Bone 79:233–241
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Tamura Y, Kawao N, Yano M, Okada K, Matsuo O, Kaji H (2014) Plasminogen activator inhibitor-1 deficiency ameliorates insulin resistance and hyperlipidemia but not bone loss in obese female mice. Endocrinology 155:1708–1717
Kawao N, Morita H, Obata K, Tamura Y, Okumoto K, Kaji H (2016) The vestibular system is critical for the changes in muscle and bone induced by hypergravity in mice. Physiol Rep 4:e12979
Pan C, Singh S, Sahasrabudhe DM, Chakkalakal JV, Krolewski JJ, Nastiuk KL (2016) TGFβ superfamily members mediate androgen deprivation therapy-induced obese frailty in male mice. Endocrinology 157:4461–4472
Wiren KM, Zhang XW, Olson DA, Turner RT, Iwaniec UT (2012) Androgen prevents hypogonadal bone loss via inhibition of resorption mediated by mature osteoblasts/osteocytes. Bone 51:835–846
Colaianni G, Mongelli T, Colucci S, Cinti S, Grano M (2016) Crosstalk between muscle and bone via the muscle-myokine irisin. Curr Osteoporos Rep 14:132–137
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM (2015) Detection and quantitation of circulating human Irisin by tandem mass spectrometry. Cell Metab 22:734–740
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Dreos R, Ambrosini G, Perier RC, Bucher P (2015) The eukaryotic promoter database: expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res 43:D92–D96
Li F, Li Y, Duan Y, Hu CA, Tang Y, Yin Y (2017) Myokines and adipokines: involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev 33:73–82
Acknowledgements
This study was partly supported by a Grant from Takeda Science Foundation and a Grant from Mitsui Life Social Welfare Foundation to N.K., a grant from Kindai Research Enhancement Grant (21st Century Joint Research Enhancement Grant) to H.K., Grants-in-Aid for Scientific Research (C:15K08220) to H.K. and (C:16K08534) to N.K., and a Grant-in-Aid for Scientific Research on Innovative Areas (Grant number 15H05935, “Living in Space”) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to H.K.
Author information
Authors and Affiliations
Contributions
SI, NK, and HK contributed to the conception and design of the research. SI, NK, and KO performed the experiments. SI and NK analyzed the data. SI, NK, MA, and HK interpreted the results of the experiments. SI and NK prepared the figures. SI drafted the manuscript. SI, NK, and HK edited and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was conducted with the approval of the Experimental Animal Welfare Committee of Kindai University (Permit number: KAME-28-016). All experimental procedures were performed according to the guidelines of the National Institutes of Health and the institutional rules for the use and care of laboratory animals at Kindai University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Iemura, S., Kawao, N., Okumoto, K. et al. Role of irisin in androgen-deficient muscle wasting and osteopenia in mice. J Bone Miner Metab 38, 161–171 (2020). https://doi.org/10.1007/s00774-019-01043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01043-7