Abstract
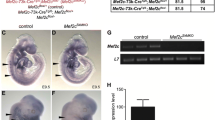
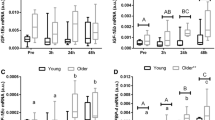
Insulin-like growth factor-I (IGF-I) is a peptide with diverse functions, among them regulation of embryonic development and bone homeostasis. Serum IGF-I levels decline in the elderly; however, IGF-I function in adults has not been clearly defined. Here, we show that IGF-I is required to maintain muscle mass in adults. We crossed Igf-I flox’d and Mx1 Cre mice to yield Mx1 Cre/Igf-Iflox/flox (IGF-I cKO) mice, and deleted Igf-I in adult mice by polyIpolyC injection. We demonstrate that, although serum IGF-I levels significantly decreased after polyIpolyC injection relative to (Igf-Iflox/flox) controls, serum glucose levels were unchanged. However, muscle mass decreased significantly after IGF-I down-regulation, while bone mass remained the same. In IGF-I cKO muscle, expression of anabolic factors such as Eif4e and p70S6K significantly decreased, while expression of catabolic factors MuRF1 and Atrogin-1 was normal and down-regulated, respectively, suggesting that observed muscle mass reduction was due to perturbed muscle metabolism. Our data demonstrate a specific role for IGF-I in maintaining muscle homeostasis in adults.





Similar content being viewed by others
References
Bartke A, Sun LY, Longo V (2013) Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 93:571–598. https://doi.org/10.1152/physrev.00006.2012
Ranke MB (2015) Insulin-like growth factor binding-protein-3 (IGFBP-3). Best Pract Res Clin Endocrinol Metab 29:701–711. https://doi.org/10.1016/j.beem.2015.06.003
Liu J-P, Baker J, Perkins AS, Roberteon EJ, Efetratiadie A (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72
DeChiara TM, Efstratiadis A, Robertson EJ (1990) A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345:76–80
Duvillié B, Cordonnier N, Deltour L, Fo Dandoy-Dron, Itier J-M, Monthioux E, Jami J, Joshi RL, Bucchini D (1997) Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci U S A 94:5137–5140
Bakker AD, Jaspers RT (2015) IL-6 and IGF-1 signaling within and between muscle and bone: how important is the mTOR pathway for bone metabolism? Curr Osteoporos Rep 13:131–139. https://doi.org/10.1007/s11914-015-0264-1
Baron R, Hesse E (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 97:311–325. https://doi.org/10.1210/jc.2011-2332
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C (2012) Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18:1095–1101. https://doi.org/10.1038/nm.2793
Wang T, Wang Y, Menendez A, Fong C, Babey M, Tahimic CG, Cheng Z, Li A, Chang W, Bikle DD (2015) Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res 30:1572–1584. https://doi.org/10.1002/jbmr.2510
Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S (2007) Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148:5706–5715. https://doi.org/10.1210/en.2007-0608
Lau KW, Rundle CH, Zhou XD, Baylink DJ, Sheng MH (2016) Conditional deletion of IGF-I in osteocytes unexpectedly accelerates bony union of the fracture gap in mice. Bone 92:18–28. https://doi.org/10.1016/j.bone.2016.08.005
Banerjee A, Guttridge DC (2012) Mechanisms for maintaining muscle (in eng). Curr Opin Support Palliat Care 6:451–456. https://doi.org/10.1097/SPC.0b013e328359b681
Wang X, Proud CG (2006) The mTOR pathway in the control of protein synthesis (in eng). Physiology (Bethesda, Md) 21:362–369. https://doi.org/10.1152/physiol.00024.2006
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy (in eng). Science (New York, NY) 294:1704–1708. https://doi.org/10.1126/science.1065874
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways (in eng). The international journal of biochemistry & cell biology 37:1974–1984. https://doi.org/10.1016/j.biocel.2005.04.018
Tando T, Hirayama A, Furukawa M, Sato Y, Kobayashi T et al (2016) Smad2/3 proteins are required for immobilization-induced skeletal muscle atrophy. J Biol Chem 291:12184–12194. https://doi.org/10.1074/jbc.M115.680579
Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice (in eng). J Biol Chem 270:12109–12116
Saber H, Himali JJ, Beiser AS, Shoamanesh A, Pikula A, Roubenoff R, Romero JR, Kase CS, Vasan RS, Seshadri S (2017) Serum Insulin-Like Growth Factor 1 and the Risk of Ischemic Stroke: the Framingham Study. Stroke 48:1760–1765. https://doi.org/10.1161/STROKEAHA.116.016563
Boguszewski CL, Boguszewski MC, Kopchick JJ (2016) Growth hormone, insulin-like growth factor system and carcinogenesis. Endokrynol Pol 67:414–426. https://doi.org/10.5603/EP.a2016.0053
Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE (2005) Molecular interactions of the IGF system. Cytokine Growth Factor Rev 16:421–439. https://doi.org/10.1016/j.cytogfr.2005.04.004
Vary TC, Jefferson LS, Kimball SR (2000) Role of eIF4E in stimulation of protein synthesis by IGF-I in perfused rat skeletal muscle (in eng). Am J Physiol Endocrinol Metab 278:E58–E64. https://doi.org/10.1152/ajpendo.2000.278.1.E58
Li M, Li C, Parkhouse WS (2002) Differential effects of des IGF-1 on Erks, AKT-1 and P70 S6K activation in mouse skeletal and cardiac muscle (in eng). Mol Cell Biochem 236:115–122
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 (in eng). Am J Physiol Endocrinol Metab 287:E591–E601. https://doi.org/10.1152/ajpendo.00073.2004
Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M (2009) Smad2 and 3 transcription factors control muscle mass in adulthood (in eng). Am J Physiol Cell Physiol 296:C1248–C1257. https://doi.org/10.1152/ajpcell.00104.2009
Kell RT, Bell G, Quinney A (2001) Musculoskeletal fitness, health outcomes and quality of life (in eng). Sports Med (Auckland, NZ) 31:863–873
Frontera WR (2017) Physiologic changes of the musculoskeletal system with aging: a brief review. Phys Med Rehabil Clin N Am 28:705–711. https://doi.org/10.1016/j.pmr.2017.06.004
Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE (1994) Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index (in eng). J Clin Endocrinol Metab 78:744–752. https://doi.org/10.1210/jcem.78.3.8126152
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis (in eng). Eur J Rheumatol 4:46–56. https://doi.org/10.5152/eurjrheum.2016.048
Hoshi H, Hao W, Fujita Y, Funayama A, Miyauchi Y et al (2012) Aldehyde-stress resulting from Aldh2 mutation promotes osteoporosis due to impaired osteoblastogenesis (in eng). J Bone Miner Res 27:2015–2023. https://doi.org/10.1002/jbmr.1634
Hofmann M, Halper B, Oesen S, Franzke B, Stuparits P, Tschan H, Bachl N, Strasser EM, Quittan M, Ploder M, Wagner KH, Wessner B (2015) Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol 64:35–45. https://doi.org/10.1016/j.exger.2015.02.008
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People (in eng). Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172. https://doi.org/10.1016/j.biocel.2013.05.036
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member (in eng). Nature 387:83–90. https://doi.org/10.1038/387083a0
Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M (2003) Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene (in eng). Genesis (New York, NY: 2000) 35:227–238. https://doi.org/10.1002/gene.10188
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene (in eng). Proc Natl Acad Sci USA 94:12457–12461
Whittemore LA, Song K, Li X, Aghajanian J, Davies M et al (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength (in eng). Biochem Biophys Res Commun 300:965–971
Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS (2002) Functional improvement of dystrophic muscle by myostatin blockade (in eng). Nature 420:418–421. https://doi.org/10.1038/nature01154
Bhattacharya I, Pawlak S, Marraffino S, Christensen J, Sherlock SP, Alvey C, Morris C, Arkin S, Binks M (2017) Safety, tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (PF-06252616), an antimyostatin monoclonal antibody, in healthy subjects. Clin Pharmacol Drug Dev. https://doi.org/10.1002/cpdd.386
Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, Lach-Trifilieff E, Roubenoff R (2017) Treatment of Sarcopenia with Bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc 65:1988–1995. https://doi.org/10.1111/jgs.14927
Goldspink DF (1977) The influence of immobilization and stretch on protein turnover of rat skeletal muscle (in eng). J Physiol 264:267–282
Howard G, Steffen JM, Geoghegan TE (1989) Transcriptional regulation of decreased protein synthesis during skeletal muscle unloading (in eng). J Appl Physiol (Bethesda, Md : 1985) 66:1093–1098. https://doi.org/10.1152/jappl.1989.66.3.1093
Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, Ohno A, Okumura Y, Nonaka I, Yasutomo K, Baldwin KM, Kominami E, Higashibata A, Nagano K, Tanaka K, Yasui N, Mills EM, Takeda S, Nikawa T (2009) Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading (in eng). Mol Cell Biol 29:4798–4811. https://doi.org/10.1128/mcb.01347-08
Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F (2010) The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis (in eng). J Endocrinol 205:201–210. https://doi.org/10.1677/joe-09-0431
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism (in eng). J Cell Biochem 116:687–695. https://doi.org/10.1002/jcb.25040
Delaney K, Kasprzycka P, Ciemerych MA, Zimowska M (2017) The role of TGF-beta1 during skeletal muscle regeneration (in eng). Cell Biol Int 41:706–715. https://doi.org/10.1002/cbin.10725
Watanabe R, Fujita N, Takeda S, Sato Y, Kobayashi T, Morita M, Oike T, Miyamoto K, Matsumoto Y, Matsumoto M, Nakamura M, Miyamoto T (2016) Ibandronate concomitantly blocks immobilization-induced bone and muscle atrophy. Biochem Biophys Res Commun 480:662–668. https://doi.org/10.1016/j.bbrc.2016.10.112
Trummer C, Schwetz V, Pandis M, Grubler MR, Verheyen N, Gaksch M, Zittermann A, Marz W, Aberer F, Lang A, Friedl C, Tomaschitz A, Obermayer-Pietsch B, Pieber TR, Pilz S, Treiber G (2017) Effects of vitamin D supplementation on IGF-1 and calcitriol: a randomized-controlled trial (in eng). Nutrients. https://doi.org/10.3390/nu9060623
Ameri P, Giusti A, Boschetti M, Bovio M, Teti C, Leoncini G, Ferone D, Murialdo G, Minuto F (2013) Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency (in eng). Eur J Endocrinol 169:767–772. https://doi.org/10.1530/eje-13-0510
Holick MF (2007) Vitamin D deficiency (in eng). N Engl J Med 357:266–281. https://doi.org/10.1056/NEJMra070553
Sakai S, Suzuki M, Tashiro Y, Tanaka K, Takeda S, Aizawa K, Hirata M, Yogo K, Endo K (2015) Vitamin D receptor signaling enhances locomotive ability in mice (in eng). J Bone Miner Res 30:128–136. https://doi.org/10.1002/jbmr.2317
Hao W, Tashiro S, Hasegawa T, Sato Y, Kobayashi T, Tando T, Katsuyama E, Fujie A, Watanabe R, Morita M, Miyamoto K, Morioka H, Nakamura M, Matsumoto M, Amizuka N, Toyama Y, Miyamoto T (2015) Hyperglycemia promotes Schwann cell de-differentiation and de-myelination via sorbitol accumulation and Igf1 protein down-regulation (in eng). J Biol Chem 290:17106–17115. https://doi.org/10.1074/jbc.M114.631291
Acknowledgements
T. Miyamoto was supported by a Grant-in-Aid for Scientific Research in Japan and a Grant from the Japan Agency for Medical Research and Development. Y. Sato, A. Kanaji, and K. Miyamoto were supported by a Grant-in-Aid for Scientific Research in Japan. This study was supported in part by a Grant-in-Aid for Scientific Research and a Grant from the Translational Research Network Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial and non-financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nakamura, S., Sato, Y., Kobayashi, T. et al. Insulin-like growth factor-I is required to maintain muscle volume in adult mice. J Bone Miner Metab 37, 627–635 (2019). https://doi.org/10.1007/s00774-018-0964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0964-6