Abstract
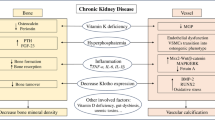
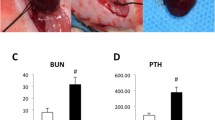
A link between vascular calcification and bone anomalies has been suggested in chronic kidney disease (CKD) patients with low bone turnover disease. We investigated the vascular expression of osteocyte markers in relation to bone microarchitecture and mineralization defects in a model of low bone turnover CKD rats with vascular calcification. CKD with vascular calcification was induced by 5/6 nephrectomy followed by high calcium and phosphate diet, and vitamin D supplementation (Ca/P/VitD). CKD + Ca/P/VitD group (n = 12) was compared to CKD + normal diet (n = 12), control + normal diet (n = 8) and control + Ca/P/VitD supplementation (n = 8). At week 6, tibia, femurs and the thoracic aorta were analysed by Micro-Ct, histomorphometry and for expression of osteocyte markers. High Ca/P/VitD treatment induced vascular calcification only in CKD rats, suppressed serum parathyroid hormone levels and led to higher sclerostin, DKK1 and FGF23 serum levels. Expression of sclerostin, DKK1 and DMP1 but not FGF23 were increased in calcified vessels from CKD + Ca/P/VitD rats. Despite low parathyroid hormone levels, tibia bone cortical thickness was significantly lower in CKD + Ca/P/VitD rats as compared to control rats fed a normal diet, which is likely the result of radial growth impairment. Finally, Ca/P/VitD treatment in CKD rats induced a bone mineralization defect, which is likely explained by the high calcitriol dose. In conclusion, Ca/P/VitD supplementation in CKD rats induces expression of osteocyte markers in vessels and bone mineralisation anomalies. Further studies should evaluate the mechanisms of high dose calcitriol-induced bone mineralisation defects in CKD.






Similar content being viewed by others
References
(2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl S1–S130 https://doi.org/10.1038/ki.2009.188
Cozzolino M, Urena-Torres P, Vervloet MG, Brandenburg V, Bover J, Goldsmith D, Larsson TE, Massy ZA, Mazzaferro S, ERA-EDTA C-MWGo (2014) Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant 29:1815–1820. https://doi.org/10.1093/ndt/gft514
Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME (1999) Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100:2168–2176
Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N, Vascular Calcification Work G (2004) Vascular calcification in chronic kidney disease. Am J Kidney Dis 43:572–579
Stehman-Breen C (2004) Osteoporosis and chronic kidney disease. Semin Nephrol 24:78–81
Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, Leonard MB, Shane E (2010) Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 21:1371–1380. https://doi.org/10.1681/ASN.2009121208
Foley RN, Parfrey PS, Sarnak MJ (1998) Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9:S16–S23
Watanabe R, Lemos MM, Carvalho AB, Rochitte CE, Santos RD, Draibe SA, Canziani ME (2012) The association between coronary artery calcification progression and loss of bone density in non-dialyzed CKD patients. Clin Nephrol 78:425–431. https://doi.org/10.5414/CN107515
Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG (2008) Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23:586–593. https://doi.org/10.1093/ndt/gfm660
Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC (2000) Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 20:1926–1931
Drueke TB, Massy ZA (2016) Changing bone patterns with progression of chronic kidney disease. Kidney Int 89:289–302. https://doi.org/10.1016/j.kint.2015.12.004
Andress DL (2008) Adynamic bone in patients with chronic kidney disease. Kidney Int 73:1345–1354. https://doi.org/10.1038/ki.2008.60
Malluche HH, Monier-Faugere MC (1992) Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl 38:S62–S67
London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC (2004) Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15:1943–1951
Frazao JM, Martins P (2009) Adynamic bone disease: clinical and therapeutic implications. Curr Opin Nephrol Hypertens 18:303–307. https://doi.org/10.1097/MNH.0b013e32832c4df0
Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D (2013) The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol 8:819–823. https://doi.org/10.2215/CJN.07670712
Ferreira JC, Ferrari GO, Neves KR, Cavallari RT, Dominguez WV, Dos Reis LM, Graciolli FG, Oliveira EC, Liu S, Sabbagh Y, Jorgetti V, Schiavi S, Moyses RM (2013) Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease—role of sclerostin? PLoS One 8:e79721. https://doi.org/10.1371/journal.pone.0079721PONE-D-13-17719
de Oliveira RA, Barreto FC, Mendes M, dos Reis LM, Castro JH, Britto ZM, Marques ID, Carvalho AB, Moyses RM, Jorgetti V (2015) Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int 87:1039–1045. https://doi.org/10.1038/ki.2014.372
Bover J, Urena P, Brandenburg V, Goldsmith D, Ruiz C, DaSilva I, Bosch RJ (2014) Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin Nephrol 34:626–640. https://doi.org/10.1016/j.semnephrol.2014.09.008
Gauthier-Bastien A, Ung RV, Lariviere R, Mac-Way F, Lebel M, Agharazii M (2013) Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypertens. https://doi.org/10.3109/10641963.2013.804541
Agharazii M, St-Louis R, Gautier-Bastien A, Ung RV, Mokas S, Lariviere R, Richard DE (2015) Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens 28:746–755. https://doi.org/10.1093/ajh/hpu225
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Chappard D, Palle S, Alexandre C, Vico L, Riffat G (1987) Bone embedding in pure methyl methacrylate at low temperature preserves enzyme activities. Acta Histochem 81:183–190. https://doi.org/10.1016/S0065-1281(87)80012-0
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610. https://doi.org/10.1002/jbmr.5650020617
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17. https://doi.org/10.1002/jbmr.1805
Moe SM (2006) Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Investig 36:51–62. https://doi.org/10.1111/j.1365-2362.2006.01665.x
Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Urena-Torres P, Bover J, Goldsmith D, ERA-EDTA C-MWGo (2014) Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2:427–436. https://doi.org/10.1016/S2213-8587(14)70059-2
Brandenburg VM, D’Haese P, Deck A, Mekahli D, Meijers B, Neven E, Evenepoel P (2015) From skeletal to cardiovascular disease in 12 steps—the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol. https://doi.org/10.1007/s00467-015-3069-7
Burgers TA, Williams BO (2013) Regulation of Wnt/beta-catenin signaling within and from osteocytes. Bone 54:244–249. https://doi.org/10.1016/j.bone.2013.02.022
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, He W (2016) WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res 345:206–217. https://doi.org/10.1016/j.yexcr.2016.06.007
Ferreira JC, Ferrari GO, Neves KR, Cavallari RT, Dominguez WV, dos Reis LM, Graciolli FG, Oliveira EC, Liu SG, Sabbagh Y, Jorgetti V, Schiavi S, Moyses RMA (2013) Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease—role of sclerostin? PLoS One 8:7. https://doi.org/10.1371/journal.pone.0079721
Carrillo-Lopez N, Panizo S, Alonso-Montes C, Roman-Garcia P, Rodriguez I, Martinez-Salgado C, Dusso AS, Naves M, Cannata-Andia JB (2016) Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int 90:77–89. https://doi.org/10.1016/j.kint.2016.01.024
Idelevich A, Kerschnitzki M, Shahar R, Monsonego-Ornan E (2011) 1,25(OH)(2)D-3 alters growth plate maturation and bone architecture in young rats with normal renal function. PLoS One 6:14. https://doi.org/10.1371/journal.pone.0020772
Hopper TAJ, Wehrli FW, Saha PK, Andre JB, Wright AC, Sanchez CP, Leonard MB (2007) Quantitative microcomputed tomography assessment of intratrabecular, intertrabecular, and cortical bone architecture in a rat model of severe renal osteodystrophy. J Comput Assist Tomogr 31:320–328. https://doi.org/10.1097/01.rct.0000238007.19258.3d
Parfitt AM (1997) The hyperparathyroidism of chronic renal failure: a disorder of growth. Kidney Int 52:3–9. https://doi.org/10.1038/ki.1997.297
Diaz MN, Rodriguez AR, Martin JLF, Arias MS, Rodriguez PM, Cannata Andia JB (2007) Effects of estradiol, calcitriol and both treatments combined on bone histomorphometry in rats with chronic kidney disease and ovariectomy. Bone 41:614–619. https://doi.org/10.1016/j.bone.2007.06.026
Wronski TJ, Halloran BP, Bikle DD, Globus RK, Morey-Holton ER (1986) Chronic administration of 1,25-dihydroxyvitamin D3: increased bone but impaired mineralization. Endocrinology 119:2580–2585. https://doi.org/10.1210/endo-119-6-2580
Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R, Carmeliet G (2012) Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Investig 122:1803–1815. https://doi.org/10.1172/JCI45890
De Schutter TM, Behets GJ, Jung S, Neven E, D’Haese PC, Querfeld U (2012) Restoration of bone mineralization by cinacalcet is associated with a significant reduction in calcitriol-induced vascular calcification in uremic rats. Calcif Tissue Int 91:307–315. https://doi.org/10.1007/s00223-012-9635-0
Wesseling-Perry K, Salusky IB (2013) Chronic kidney disease: mineral and bone disorder in children. Semin Nephrol 33:169–179. https://doi.org/10.1016/j.semnephrol.2012.12.017
Newman CL, Tian N, Hammond MA, Wallace JM, Brown DM, Chen NX, Moe SM, Allen MR (2016) Calcitriol suppression of parathyroid hormone fails to improve skeletal properties in an animal model of chronic kidney disease. Am J Nephrol 43:20–31. https://doi.org/10.1159/000444423
Fortier C, Mac-Way F, De Serres SA, Marquis K, Douville P, Desmeules S, Lariviere R, Agharazii M (2014) Active vitamin D and accelerated progression of aortic stiffness in hemodialysis patients: a longitudinal observational study. Am J Hypertens 27:1346–1354. https://doi.org/10.1093/ajh/hpu057
Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM (2011) Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 26:1425–1436. https://doi.org/10.1002/jbmr.345
Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG (2011) Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int 89:140–150. https://doi.org/10.1007/s00223-011-9501-5
Jung S, Querfeld U, Muller D, Rudolph B, Peters H, Kramer S (2012) Submaximal suppression of parathyroid hormone ameliorates calcitriol-induced aortic calcification and remodeling and myocardial fibrosis in uremic rats. J Hypertens 30:2182–2191. https://doi.org/10.1097/HJH.0b013e328357c049
Acknowledgements
Dr. Mac-Way holds a scholarship from FRQ-S (Grant no. 32661) and KRESCENT program from the CIHR-Kidney Foundation of Canada. This study was supported by a grant from the Kidney Foundation of Canada (Grant no. KFOC160013) and from the Department of Medicine and la Fondation du CHU de Québec from Université Laval. Dr. Agharazii holds a research chair in nephrology from Université Laval.
Author information
Authors and Affiliations
Contributions
MA, RL and FMW conceived and designed the study. SKB and FMW performed the statistical analyses. SKB, DV and RVU conducted animal experimentations, tissue specimens handling, and interpreted the findings. SP and FMW were in charge of the bone histomorphometry analysis. SKB and FMW wrote the first draft and revised all subsequent versions. All authors provided their input, expertise and critical review of the paper. All authors read and approved the final version of the paper. FMW had full access to the data and takes responsibility for the integrity and accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Bisson, SK., Ung, RV., Picard, S. et al. High calcium, phosphate and calcitriol supplementation leads to an osteocyte-like phenotype in calcified vessels and bone mineralisation defect in uremic rats. J Bone Miner Metab 37, 212–223 (2019). https://doi.org/10.1007/s00774-018-0919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0919-y