Summary.
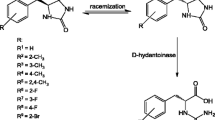
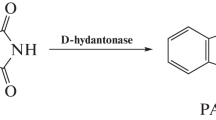
d-Hydantoinase from Vigna angularis hydrolyzed rac-5-monosubstituted-hydantoins with polar and aromatic side chains and dihydrothymine but rac-5,5-disubstituted-hydantoins were not substrates of this enzyme. 5-Phenylhydantoin was the best substrate. By using this substrate, N-carbamoyl-d-phenylglycine was obtained in quantitative yield and over 98% ee.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received February 17, 2000; Accepted April 4, 2000
Rights and permissions
About this article
Cite this article
Arcuri, M., Antunes, O., Sabino, S. et al. Resolution of dl-hydantoins by d-hydantoinase from Vigna angularis: Production of highly enantioenriched N-carbamoyl-d-phenylglycine at 100% conversion. Amino Acids 19, 477–482 (2000). https://doi.org/10.1007/s007260070025
Issue Date:
DOI: https://doi.org/10.1007/s007260070025