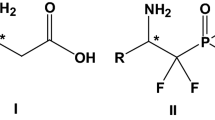
Abstract
α-Amino acids (α-AAs) are in extremely high demand in nearly every sector of the food and health-related chemical industries and continue to be the subject of intense multidisciplinary research. The self-disproportionation of enantiomers (SDE) is an emerging and one of the least studied areas of α-AA or enantiomeric properties, critically important for their production and application. In the present work, we report a detailed study of the SDE via achiral, gravity-driven column chromatography for a set of N-acylated, N-carbonylated, N-fluoroacylated, and N-thioacylated α-amino acid esters. As well as thioacylation, attention was paid to the effect of altering the R group of the ester functionality, the side chain, or that of the acyl group attached to the amide nitrogen, whereby it was found that electron-withdrawing groups in the latter moiety had a pronounced effect on the magnitude and behavior of the resulting SDE phenomenon. Intriguingly, in the case of N-fluoroacylated derivatives, by favoring the formation of dimeric associates and effecting a strong bias toward homochiral associates over heterochiral associates, the SDE magnitude was greatly reduced contrary to intuitive expectations. Energy estimates resulted from DFT calculations.






Similar content being viewed by others
References
Abás S, Arróniz C, Molins E, Escolano C (2018) Access to the enantiopure pyrrolobenzodiazepine (PBD) dilactam nucleus via self-disproportionation of enantiomers. Tetrahedron 74:867–871
Aceña JL, Sorochinsky AE, Moriwaki H, Sato T, Soloshonok VA (2013a) Synthesis of fluorine-containing α-amino acids in enantiomerically pure form via homologation of Ni(II) complexes of glycine and alanine Schiff bases. J Fluor Chem 155:21–38
Aceña JL, Sorochinsky AE, Katagiri T, Soloshonok VA (2013b) Unconventional preparation of racemic crystals of isopropyl 3,3,3-trifluoro-2-hydroxypropanoate and their unusual crystallographic structure: the ultimate preference for homochiral intermolecular interactions. Chem Commun 49:373–375
Aceña JL, Sorochinsky AE, Soloshonok VA (2014) Asymmetric synthesis of α-amino acids via homologation of Ni(II) complexes of glycine Schiff bases. Part 3: michael addition reactions and miscellaneous transformations. Amino Acids 46:2047–2073
Baciocchi R, Zenoni G, Mazzotti M, Morbidelli M (2002) Separation of binaphthol enantiomers through achiral chromatography. J Chromatogr A 944:225–240
Baciocchi R, Mazzotti M, Morbidelli M (2004) General model for the achiral chromatography of enantiomers forming dimers: application to binaphthol. J Chromatogr A 1024:15–20
Bravo P, Farina A, Frigerio M, Valdo Meille S, Viani F, Soloshonok VA (1994) New fluorinated chiral synthons. Tetrahedron Asymmetry 5:987–1004
Bravo P, Farina A, Kukhar VP, Markovsky AL, Meille SV, Soloshonok VA, Sorochinsky AE, Viani F, Zanda M, Zappala C (1997) Stereoselective additions of α-lithiated alkyl-p-tolylsulfoxides to N-PMP(fluoroalkyl)aldimines. An efficient approach to enantiomerically pure fluoro amino compounds. J Org Chem 62:3424–3425
Bravo P, Guidetti M, Viani F, Zanda M, Markovsky AL, Sorochinsky AE, Soloshonok IV, Soloshonok VA (1998) Chiral sulfoxide controlled asymmetric additions to C–N double bond. An efficient approach to stereochemically defined α-fluoroalkyl amino compounds. Tetrahedron 54:12789–12806
Drabowicz J, Jasiak A, Wzorek A, Sato A, Soloshonok VA (2017) Self-disproportionation of enantiomers (SDE) of chiral sulfoxides, amides and thioamides via achiral chromatography. Arkivoc 2017:557–578
Ellis TK, Ueki H, Yamada T, Ohfune Y, Soloshonok VA (2006) Design, synthesis, and evaluation of a new generation of modular nucleophilic glycine equivalents for the efficient synthesis of sterically constrained α-amino acids. J Org Chem 71:8572–8578
Gibson SE, Guillo N, Tozer MJ (1999) Towards control of χ-space: conformationally constrained analogues of Phe, Tyr, Trp and His. Tetrahedron 55:585–615
Gil-Av E, Schurig V (1994) Resolution of non-racemic mixtures in achiral chromatographic systems: a model for the enantioselective effects observed. J Chromatogr A 666:519–525
Han J, Nelson DJ, Sorochinsky AE, Soloshonok VA (2011) Self-disproportionation of enantiomers via sublimation; new and truly green dimension in optical purification. Curr Org Synth 8:310–317
Han J, Soloshonok VA, Klika KD, Drabowicz J, Wzorek A (2018a) Chiral sulfoxides: advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem Soc Rev 47:1307–1350
Han J, Kitagawa O, Wzorek A, Klika KD, Soloshonok VA (2018b) The self-disproportionation of enantiomers (SDE): a menace or an opportunity? Chem Sci 9:1718–1739
He G, Wang B, Nack WA, Chen G (2016) Syntheses and transformations of α-amino acids via palladium-catalyzed auxiliary-directed sp3 C-H functionalization. Acc Chem Res 49:635–645
Hodgson DRW, Sanderson JM (2004) The synthesis of peptides and proteins containing non-natural amino acids. Chem Soc Rev 33:422–430
Jung M, Schurig V (1992) Computer simulation of three scenarios for the separation of non-racemic mixtures by chromatography on achiral stationary phases. J Chromatogr 605:161–166
Klika KD, Budovská M, Kutschy P (2010) NMR spectral enantioresolution of spirobrassinin and 1-methoxyspirobrassinin enantiomers using (S)-(-)-ethyl lactate and modeling of spirobrassinin self-association for rationalization of its self-induced diastereomeric anisochronism (SIDA) and enantiomer self-disproportionation on achiral-phase chromatography (ESDAC) phenomena. J Fluor Chem 131:467–476
Kukhar VP, Sorochinsky AE, Soloshonok VA (2009) Practical synthesis of fluorine-containing α- and β-amino acids: recipes from Kiev, Ukrain. Future Med Chem 1:793–819
Ma JS (2003) Unnatural amino acids in drug discovery. Chim Oggi/Chem Today 21:65–68
Maeno M, Tokunaga E, Yamamoto T, Suzuki T, Ogino Y, Ito E, Shiro M, Asahi T, Shibata N (2015) Self-disproportionation of enantiomers of thalidomide and its fluorinated analogue via gravity-driven achiral chromatography: mechanistic rationale and implications. Chem Sci 6:1043–1048
Martens J, Bhushan R (1992) Resolution of enantiomers with achiral phase chromatography. J Liq Chromatogr Relat Technol 15:1–27
Martens J, Bhushan R (2014) Purification of enantiomeric mixtures in enantioselective synthesis: overlooked errors and scientific basis of separation in achiral environment. Helv Chim Acta 97:161–187
Martens J, Bhushan R (2016) Enantioseparations in achiral environments and chromatographic systems. Isr J Chem 56:990–1009
Mayani VJ, Abdi SHR, Kureshy RI, Khan NH, Agrawal S, Jasra RV (2009) Enantiomer self-disproportionation of chiral compounds on achiral ordered mesoporous silica M41S and regular silica gel as a stationary phase. Chirality 21:255–261
Metz AE, Kozlowski MC (2015) Recent advances in asymmetric catalytic methods for the formation of acyclic α,α-disubstituted α-amino acids. J Org Chem 80:1–7
Mikami K, Fustero S, Sánchez-Roselló M, Aceña JL, Soloshonok VA, Sorochinsky AE (2011) Synthesis of fluorinated β-amino acids. Synthesis 2011:3045–3079
Mikhailiuk PK, Afonin S, Chernega AN, Rusanov EB, Platonov MO, Dubinina GG, Berditsch M, Ulrich AS, Komarov IV (2006) Conformationally rigid trifluoromethyl-substituted α-amino acid designed for peptide structure analysis by solid-state 19F NMR spectroscopy. Angew Chem Int Ed 45:5659–5661
Monde K, Harada N, Takasugi M, Kutschy P, Suchý M, Dzurilla M (2000) Enantiomeric excess of a cruciferous phytoalexin, spirobrassinin, and its enantiomeric enrichment in an achiral HPLC system. J Nat Prod 63:1312–1314
Nakamura T, Tateishi K, Tsukagoshi S, Hashimoto S, Watanabe S, Soloshonok VA, Aceña JL, Kitagawa O (2012) Self-disproportionation of enantiomers of non-racemic chiral amine derivatives through achiral chromatography. Tetrahedron 68:4013–4017
Ogawa S, Nishimine T, Tokunaga E, Nakamura S, Shibata N (2010) Self-disproportionation of enantiomers of heterocyclic compounds having a tertiary trifluoromethyl alcohol center on chromatography with a non-chiral system. J Fluor Chem 131:521–524
Reyes-Rangel G, Vargas-Caporali J, Juaristi E (2017) Asymmetric Michael addition reaction organocatalyzed by stereoisomeric pyrrolidine sulfinamides under neat conditions. A brief study of self-disproportionation of enantiomers. Tetrahedron 73:4707–4718
Sato T, Izawa K, Aceña JL, Liu H, Soloshonok VA (2016) Tailor-made α-amino acids in the pharmaceutical industry: synthetic approaches to (1R,2S)-1-amino-2-vinylcyclopropane-1-carboxylic acid (vinyl-ACCA). Eur J Org Chem 2016:2757–2774
Schurig V (2009) Elaborate treatment of retention in chemoselective chromatography—the retention increment approach and nonlinear effects. J Chromatogr A 1216:1723–1736
So SM, Kim H, Mui L, Chin J (2012) Mimicking nature to make unnatural amino acids and chiral diamines. Eur J Org Chem 2012:229–241
Soloshonok VA (2002) Highly diastereoselective Michael addition reactions between nucleophilic glycine equivalents and β-substituted-α,β-unsaturated carboxylic acid derivatives a general approach to the stereochemically defined and sterically χ-constrained & α-amino acids. Curr Org Chem 6:341–364
Soloshonok VA (2006) Remarkable amplification of the self-disproportionation of enantiomers on achiral-phase chromatography columns. Angew Chem Int Ed 45:766–769
Soloshonok VA, Berbasov DO (2006a) Self-disproportionation of enantiomers on achiral phase chromatography. One more example of fluorine’s magic powers. Chim Oggi Chem Today 24:44–47
Soloshonok VA, Berbasov DO (2006b) Self-disproportionation of enantiomers of (R)-ethyl 3-(3,5-dinitrobenzamido)-4,4,4-trifluorobutanoate on achiral silica gel stationary phase. J Fluor Chem 127:597–603
Soloshonok VA, Ono T (1996) The effect of substituents on the feasibility of azomethine–azomethine isomerization: new synthetic opportunities for biomimetic transamination. Tetrahedron 52:14701–14712
Soloshonok VA, Sorochinsky AE (2010) Practical methods for the synthesis of symmetrically αα-disubstituted α-amino acids. Synthesis 2010:2319–2344
Soloshonok VA, Kirilenko AG, Kukhar VP, Resnati G (1993a) Transamination of fluorinated β-keto carboxylic esters. A biomimetic approach to β-polyfluoroalkyl-β-amino acids. Tetrahedron Lett 34:3621–3624
Soloshonok VA, Kukhar VP, Galushko SV, Svistunova NY, Avilov DV, Kuzmina NA, Raevski NI, Struchkov YT, Pisarevsky AP, Belokon YN (1993b) General method for the synthesis of enantiomerically pure β-hydroxy-α-amino acids, containing fluorine atoms in the side chains. Case of stereochemical distinction between methyl and trifluoromethyl groups. X-Ray crystal and molecular structure of the nickel(II) complex of (2S,3S)-2(trifluoromethyl)threonine. J Chem Soc Perkin Trans 1:3143–3155
Soloshonok VA, Avilov DV, Kukhar VP (1996a) Asymmetric aldol reactions of trifluoromethyl ketones with a chiral Ni(II) complex of glycine: stereocontrolling effect of the trifluoromethyl group. Tetrahedron 52:12433–12442
Soloshonok VA, Avilov DV, Kukhar VP (1996b) Highly diastereoselective asymmetric aldol reactions of chiral Ni(II)-complex of glycine with alkyl trifluoromethyl ketones. Tetrahedron Asymmetry 7:1547–1550
Soloshonok VA, Cai C, Hruby VJ, Meervelt LV, Mischenko N (1999) Stereochemically defined C-substituted glutamic acids and their derivatives. 1. An efficient asymmetric synthesis of (2S,3S)-3-methyl- and -3-trifluoromethylpyroglutamic acids. Tetrahedron 55:12031–12044
Soloshonok VA, Ueki H, Ellis TK, Yamada T, Ofhune Y (2005) Application of modular nucleophilic glycine equivalents for truly practical asymmetric synthesis of β-substituted pyroglutamic acids. Tetrahedron Lett 46:1107–1110
Soloshonok VA, Roussel Ch, Kitagawa O, Sorochinsky AE (2012) Self-disproportionation of enantiomers via achiral chromatography: a warning and an extra dimension in optical purifications. Chem Soc Rev 41:4180–4188
Soloshonok VA, Wzorek A, Klika KD (2017) A question of policy: should tests for the self-disproportionation of enantiomers (SDE) be mandatory for reports involving scalemates? Tetrahedron Asymmetry 28:1430–1434
Sorochinsky AE, Soloshonok VA (2013) In Self-disproportionation of enantiomers of enantiomerically enriched compounds in topics in current chemistry. In: Schurig V (ed) Differentiation of enantiomers II, vol 341. Springer-Verlag GmbH, Berlin, pp 301–340
Sorochinsky AE, Aceña JL, Moriwaki H, Sato T, Soloshonok VA (2013a) Asymmetric synthesis of α-amino acids via homologation of Ni(II) complexes of glycine Schiff bases; Part 1: alkyl halide alkylations. Amino Acids 45:691–718
Sorochinsky AE, Aceña JL, Moriwaki H, Sato T, Soloshonok VA (2013b) Asymmetric synthesis of α-amino acids via homologation of Ni(II) complexes of glycine Schiff bases. Part 2: aldol, Mannich addition reactions, deracemization and (S) to (R) interconversion of α-amino acids. Amino Acids 45:1017–1033
Sorochinsky AE, Aceña JL, Soloshonok VA (2013c) Self-disproportionation of enantiomers of chiral, non-racemic fluoroorganic compounds: role of fluorine as enabling element. Synthesis 45:141–152
Sorochinsky AE, Katagiri T, Ono T, Wzorek A, Aceña JL, Soloshonok VA (2013d) Optical purifications via self-disproportionation of enantiomers by achiral chromatography: case study of a series of α-CF3-containing secondary alcohols. Chirality 25:365–368
Suchý M, Kutschy P, Monde K, Goto H, Harada N, Takasugi M, Dzurilla M, Balentová E (2001) Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindole phytoalexin, (S)-(−)-spirobrassinin, and its oxazoline analog. J Org Chem 66:3940–3947
Suzuki Y, Han J, Kitagawa O, Aceña JL, Klika KD, Soloshonok VA (2015) A comprehensive examination of the self-disproportionation of enantiomers (SDE) of chiral amides via achiral, laboratory-routine, gravity-driven column chromatography. RSC Adv 5:2988–2993
Ueki H, Yasumoto M, Soloshonok VA (2010) Rational application of self-disproportionation of enantiomers via sublimation—a novel methodological dimension for enantiomeric purifications. Tetrahedron Asymmetry 21:1396–1400
Urman S, Gaus K, Yang Y, Strijowski U, Sewald N, De Pol S, Reiser O (2007) The constrained amino acid & β-Acc confers potency and selectivity to integrin ligands. Angew Chem Int Ed 46:3976–3978
Wzorek A, Klika KD, Drabowicz J, Sato A, Aceña JL, Soloshonok VA (2014) The self-disproportionation of the enantiomers (SDE) of methyl n-pentyl sulfoxide via achiral, gravity-driven column chromatography: a case study. Org Biomol Chem 12:4738–4746
Wzorek A, Sato A, Drabowicz J, Soloshonok VA, Klika KD (2015) Enantiomeric enrichments via the self-disproportionation of enantiomers (SDE) by achiral, gravity-driven column chromatography: a case study using N-(1-phenylethyl)acetamide for optimizing the enantiomerically pure yield and magnitude of the SDE. Helv Chem Acta 98:1147–1159
Wzorek A, Sato A, Drabowicz J, Soloshonok VA (2016a) Self-disproportionation of enantiomers via achiral gravity-driven column chromatography: a case study of N-acyl-α-phenylethylamines. J Chromatogr A 1467:270–278
Wzorek A, Sato A, Drabowicz J, Soloshonok VA (2016b) Self-disproportionation of enantiomers (SDE) of chiral nonracemic amides via achiral chromatography. Isr J Chem 56:977–989
Wzorek A, Sato A, Drabowicz J, Soloshonok VA, Klika KD (2016c) Remarkable magnitude of the self-disproportionation of enantiomers (SDE) via achiral chromatography: application to the practical-scale enantiopurification of β-amino acid esters. Amino Acids 48:605–613
Wzorek A, Kamizela A, Sato A, Soloshonok VA (2017) Self-disproportionation of enantiomers (SDE) via achiral gravity-driven column chromatography of N-fluoroacyl-1-phenylethylamines. J Fluor Chem 196:37–43
Yasumoto M, Ueki H, Soloshonok VA (2010) Self-disproportionation of enantiomers of 3,3,3-trifluorolactic acid amides via sublimation. J Fluor Chem 131:266–269
Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Science and Higher Education, Poland (Grant no. 612 512, AW) and IKERBASQUE, the Basque Foundation for Science, Spain (VAS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Additional information
Handling Editor: P. Meffre.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosaka, T., Imai, T., Wzorek, A. et al. The self-disproportionation of enantiomers (SDE) of α-amino acid derivatives: facets of steric and electronic properties. Amino Acids 51, 283–294 (2019). https://doi.org/10.1007/s00726-018-2664-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2664-x