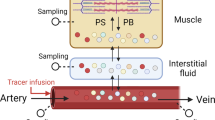
Abstract
Traditionally, the effect of dietary lysine upon health is determined through the concentrations of plasma proteins, but sometimes they are not responsive to lysine intake. We hypothesized that the fractional synthesis rates (FSRs) of plasma proteins may be more sensitive to dietary intake of lysine than protein concentrations in plasma. Seventy-two male Sprague–Dawley rats were divided randomly into three groups based on their diets provided for 18 weeks: low lysine (LG), normal lysine (NG) and high lysine (HG). Rats underwent labeling with deuterated water, a more reliable tracer than amino-acid tracers. The FSRs of albumin and immunoglobulin (Ig) G in plasma increased with increasing dietary intake of lysine. However, the albumin concentration in plasma in rats in the LG did not decrease significantly compared with that in the NG, and a similar result was shown for the IgG concentration between the NG and HG. These results suggested that the FSRs of albumin and IgG in plasma were more sensitive to dietary intake of lysine than their concentrations, and could be useful as sensitive indicators of the effect of dietary lysine upon health.

Similar content being viewed by others
References
Belloto E, Fdr Diraison, Basset A, Allain G, Abdallah P, Beylot M (2007) Determination of protein replacement rates by deuterated water: validation of underlying assumptions. Am J Physiol Endocrinol Metab 292:1340–1347
Bonjour J (2005) Dietary protein: an essential nutrient for bone health. J Am Coll Nutr 24:526–536
Busch R et al (2006) Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochem Biophys Acta 1760:730–744
Canfield LM, Chytil F (1978) Effect of low lysine diet on rat protein metabolism. J Nutr 108:1343–1347
Chen Y-Y, Lin S-Y, Yeh Y-Y, Hsiao H-H, Wu C-Y, Chen S-T, Wang AH-J (2005) A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis 26:2117–2127
Debro J, Korner A (1956) Solubility of albumin in alcohol after precipitation by trichloroacetic acid: a simplified procedure for separation of albumin. Nature 178:1067
Dufner DA et al (2005) Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab 288:1277–1283
Gasier HG, Riechman SE, Wiggs MP, Previs SF, Fluckey JD (2009) A comparison of 2H2O and phenylalanine flooding dose to investigate muscle protein synthesis with acute exercise in rats. Am J Physiol Endocrinol Metab 297:252–259
Gasier HG, Fluckey JD, Previs SF (2010) The application of 2H2O to measure skeletal muscle protein synthesis. Nutr Metab 7:31–38
Ghosh S, Pellett PL, Aw-Hassan A, Mouneime Y, Smriga M, Scrimshaw NS (2008) Impact of lysine-fortified wheat flour on morbidity and immunologic variables among members of rural families in northwest Syria. Food Nutr Bull 29:163–171
Gietzen D, Rogers Q (2006) Nutritional homeostasis and indispensable amino acid sensing: a new solution to an old puzzle. Trends Neurosci 29:91–99
Goto S, Nagao K, Bannai M, Takahashi M, Nakahara K, Kangawa K, Murakami N (2010) Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience 166:333–340
Hao S et al (2005) Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307:1776–1778
Holm L et al (2013) Determination of steady-state protein breakdown rate in vivo by the disappearance of protein-bound tracer-labeled amino acids: a method applicable in humans. Am J Physiol Endocrinol Metab 304:895–907
Hrupka BJ, Lin Y, Gietzen DW, Rogers QR (1999) Lysine deficiency alters diet selection without depressing food intake in rats. J Nutr 129:424–430
Huang L et al (2011) Lysine requirement of the enterally fed term infant in the first month of life. Am J Clin Nutr 94:1496–1503
Jackson AA, Phillips G, Mcclelland I, Jahoor F (2001) Synthesis of hepatic secretory proteins in normal adults consuming a diet marginally adequate in protein. Am J Physiol Gastrointest Liver Physiol 281:1179–1187
James WP, Hay AM (1968) Albumin metabolism: effect of the nutritional state and the dietary protein intake. J Clin Invest 47:1958–1972
Jeffay H, Winzler RJ (1958) The metabolism of serum proteins. II. The effect of dietary protein on the turnover of rat serum protein. J Biol Chem 231:111–116
Khan L, Bamji M (1979) Tissue carnitine deficiency due to dietary lysine deficiency: triglyceride accumulation and concomitant impairment in fatty acid oxidation. J Nutr 109:24–31
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Nagao K, Bannai M, Seki S, Kawai N, Mori M, Takahashi M (2010) Voluntary wheel running is beneficial to the amino acid profile of lysine-deficient rats. Am J Physiol Endocrinol Metab 298:1170–1178
Nielsen K, Nansen P (1967) Metabolism of bovine immunoglobulin II. Metabolism of bovine igg in cattle with secondary hypoimmunoglobulinemia. Can J Comp Med Vet Sci 31:106–110
Petro TM, Bhattacharjee JK (1980) Effect of dietary essential amino acid limitations upon native levels of murine serum immunoglobulins, transferrin, and complement. Infect Immun 27:513–518
Pillai RR, Elango R, Ball RO, Kurpad AV, Pencharz PB (2015) Lysine requirements of moderately undernourished school-aged indian children are reduced by treatment for intestinal parasites as measured by the indicator amino acid oxidation technique. J Nutr 145:954–959
Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR (2004) Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab 286:665–672
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Regmi N, Wang T, Crenshaw M, Rude BJ, Liao SF (2017) Effects of dietary lysine levels on the concentrations of selected nutrient metabolites in blood plasma of late-stage finishing pigs. J Anim Physiol Anim Nutr 102:1–12
Schwert GW (1957) Recovery of native bovine serum albumin after precipitation with trichloroacetic acid and solution in organic solvents. J Am Chem Soc 79:139–141
Shimomura A et al (2014) Dietary l-lysine prevents arterial calcification in adenine-induced uremic rats. J Am Soc Nephrol 25:1954–1965
Smriga M, Kameishi M, Uneyama H, Torii K (2002) Dietary l-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. J Nutr 132:3744–3746
Volpi E et al (1996) Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes 45:1245–1252
Wada Y, Sato Y, Miyazakia K, Takedaa Y, Kuwahatac M (2017) The reduced/oxidized state of plasma albumin is modulated by dietary protein intake partly via albumin synthesis rate in rats. Nutr Res 37:46–57
Wei Z, Peebles E, Wang X, Gerard P, Olanrewaju H, Mercier Y (2016) Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (III): serum metabolites, hormones, and their relationship with growth performance. J Appl Poultry Res 25:223–231
Wen Z, Rasolofomanana T, Tang J, Jiang Y, Xie M, Yang P, Hou S (2017) Effects of dietary energy and lysine levels on growth performance and carcass yields of Pekin ducks from hatch to 21 days of age. Poult Sci 96:3361–3366
Wilson W, Roach P (2002) Nutrient-regulated protein kinases in budding yeast. Cell 111:155–158
Zhao W et al (2004) Lysine-fortified wheat flour improves the nutritional and immunological status of wheat-eating families in northern China. Food Nutr Bull 25:123–129
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81472963). The authors are very grateful to Professor Xiaoguang Yang (National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention) for his invaluable suggestions on study design, and to Dr. Juan Liu (Analysis Center of Yangzhou University) for her skilled technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Animal Care and Use Committees of Yangzhou University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling Editor: J. M. Phang.
Rights and permissions
About this article
Cite this article
Tian, Y., Shi, M., Dai, Q. et al. The fractional synthesis rates of plasma proteins as determined using deuterated water are sensitive to dietary intake of lysine in rats. Amino Acids 50, 1719–1727 (2018). https://doi.org/10.1007/s00726-018-2645-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2645-0