Abstract
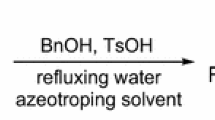
The simplest way to prepare the tosylate salts of amino acid benzyl esters, whose enantiomers are very important synthetic intermediates, is treatment of amino acid with benzyl alcohol and p-toluenesulfonic acid in a refluxing water-azeotroping solvent (Fischer–Speier esterification). However, to this day, the literature proposes only hazardous solvents, such as benzene, carbon tetrachloride, and chloroform, which must be absolutely avoided, or solvents, such as toluene and benzyl alcohol, which cause racemization because of too high boiling water azeotropes. On the other hand, the alternative successful use of cyclohexane, which we have recently reported for several amino acid benzyl esters, is inapplicable or not very efficient for ‘problematic’ amino acid such as tryptophan, arginine, and methionine, for which, indeed, the simple Fischer–Speier esterification is not described or poorly exemplified in the literature. Therefore, more polar solvents, in particular the green ethers CPME, TAME, and Me-THF, were selected and first considered for the preparation of methionine benzyl ester, previously accomplished in cyclohexane with modest yield. After discarding CPME and TAME, because causing racemization and decomposing under acidic conditions, respectively, we focused on Me-THF. In this ether, the benzyl esters of Met, Arg, and Trp could be obtained in good yield and, as proved by chiral HPLC or H NMR analysis, enantiomerically pure. The procedure was successfully extended to proline benzyl ester, which could be prepared enantiomerically pure and in quantitative yield both in cyclohexane and in Me-THF, thus avoiding the recently reported use of carbon tetrachloride.



Similar content being viewed by others
References
Adams R, Fleš D (1959) The absolute configuration of the C8-atom in the pyrrolizidine moieties of the Senecio alkaloids. J Am Chem Soc 81:5803–5805
Arai I, Muramatsu I (1983) A simple and convenient method for esterification of tryptophan and other amino acids. J Org Chem 48:121–123
Aul R, Comanita B (2007) A green alternative to THF. Manuf Chem 78:33–34
Aycock DF (2007) Solvent applications of 2-methyltetrahydrofuran in organometallic and biphasic reactions. Org Process Res Dev 11:156–159
Azzena U, Carraro M, Pisano L (2015) Un’alternativa “green” al toluene. La Chimica e l’Industria 97:28–30
Biondini D, Brinchi L, Germani R, Goracci L, Savelli G (2010) Esterification of unprotected α-amino acids in ionic liquids as the reaction media. Lett Org Chem 7:39–44
Bolchi C, Pallavicini M, Rusconi C, Diomede L, Ferri N, Corsini A, Fumagalli L, Pedretti A, Vistoli G, Valoti E (2007) Peptidomimetic inhibitors of farnesyltransferase with high in vitro activity and significant cellular potency. Bioorg Med Chem Lett 17:6192–6196
Bolchi C, Pallavicini M, Fumagalli L, Ferri N, Corsini A, Rusconi C, Valoti E (2009) New Ras CAAX mimetics: design, synthesis, antiproliferative activity, and RAS prenylation inhibition. Bioorg Med Chem Lett 19:5500–5504
Bolchi C, Valoti E, Fumagalli L, Straniero V, Ruggeri P, Pallavicini M (2015a) Enantiomerically pure dibenzyl esters of l-aspartic and l-glutamic acid. Org Process Res Dev 19:878–883
Bolchi C, Valoti E, Gotti C, Fasoli F, Ruggeri P, Fumagalli L, Binda M, Mucchietto V, Sciaccaluga M, Budriesi R, Fucile S, Pallavicini M (2015b) Chemistry and pharmacology of a series of unichiral analogues of 2-(2-pyrrolidinyl)-1,4-benzodioxane, prolinol phenyl ether, and prolinol 3-pyridyl ether designed as α4β2-nNicotinic acetylcholine receptor agonists. J Med Chem 58:6665–6677
Bolchi C, Bavo F, Pallavicini M (2017a) One-step preparation of enantiopure l- or d-amino acid benzyl esters avoiding the use of banned solvents. Amino Acids 49:965–974
Bolchi C, Bavo F, Pallavicini M (2017b) Phase diagrams to evaluate the opportunity for enantiomeric enrichment of some nonracemic mixtures of amino acid benzyl esters by crystallization as p-toluenesulfonate salts. Org Process Res Dev 21:1752–1757
Bräuer TM, Zhang Q, Tiefenbacher K (2016) Iminium catalysis inside a self-assembled supramolecular capsule: modulation of enantiomeric excess. Angew Chem Int Ed 55:7698–7701
Byrne FP, Jin S, Paggiola G, Petchey THM, Clark JH, Farmer TJ, Hunt AJ, McElroy CR, Sherwood J (2016) Tools and techniques for solvent selection: green solvent selection guides. Sustain Chem Process 4:1–24
Cerić H, Šindler-Kulyk M, Kovačević M, Perić M, Živković A (2010) Azetidinone-isothiazolidinones: stereoselective synthesis and antibacterial evaluation of new monocyclic beta-lactams. Bioorg Med Chem 18:3053–3058
Dai N, Etzkorn FA (2009) Cis-trans proline isomerization effects on collagen triple-helix stability are limited. J Am Chem Soc 131:13728–13732
Dorman L C, Cheng R C (1976) Fibrinogen peptide derivatives. US 3966701
Dwyer DS (2005) Electronic properties of amino acid side chains: quantum mechanics calculation of substituent effects. BMC Chem Biol 5:2
Fayad AA, Pubill-Ulldemolins C, Sharma SV, Day D, Goss RJM (2015) A one-pot synthesis of symmetrical and unsymmetrical dipeptide ureas. Eur J Org Chem 2015:5603–5609
Hwu JR, Jain ML, Tsai FY, Balakumar A, Hakimelahi GH, Tsay SC (2002) Ceric ammonium nitrated impregnated on silica gel in the removal of the tert-butoxycarbonyl group. Arkivoc ix:26–28
Izumiya N, Makisumi S (1957) Synthesis of aminoacid benzyl ester p-toluenesulfonates. Nippon Kagaku Zasshi 78:662–664
Kawasaki K, Kawasaki C, Maeda M, Okada Y (1980) Synthesis of tetradecapeptide corresponding to sequence 90-103 of bovine adrenodoxin. Chem Pharm Bull 28:2105–2115
Liu J, Wang L (2017) Recent advances in asymmetric reactions catalyzed by proline and its derivatives. Synthesis 49:960–972
Liu J, Wu G, Cui G, Wang W-X, Zhao M, Wang C, Zhang Z, Peng S (2007) A new class of anti-thrombosis hexahydropyrazino-[1′,2′:1,6]pyrido-[3,4b]-indole-1,4-dions: design, synthesis, logK determination, and QSAR analysis. Bioorg Med Chem 15:5672–5693
Liu J, Zhao M, Qian K, Zhang X, Lee K-H, Wu J, Liu Y-N, Peng S (2010) Benzyl 1,2,3,5,11,11a-hexahydro-3,3-dimethyl-1-oxo-6H-imidazo[3′,4′:1,2]pyridine[3,4-b]indole-2-substituted acetates: one-pot-preparation, anti-tumor activity, docking toward DNA and 3D QSAR analysis. Bioorg Med Chem 18:1910–1917
Magnus P, Ladlow M, Kim CS, Boniface P (1989) Use of the Barton decarboxylation procedure in indole alkaloid chemistry. Heterocycles 28:951–956
Mao Z-Y, Geng H, Zhang T-T, Ruan Y-P, Ye J-L, Huang P-Q (2016) Stereodivergent and enantioselective total syntheses of isochaetominines A-C and four pairs of isochaetominine C enantiomers: a six-step approach. Org Chem Front 3:24–37
Martin C, Lebrun A, Martinez J, Cavelier F (2013) Synthesis of homopolypeptides with PPII structure. J Polym Sci Part A Polym Chem 51:3103–3109
Neuman RE, Smith EL (1951) Synthesis of proline and hydroxyproline peptides; their cleavage by prolinase. J Biol Chem 193:97–111
Otsuka H, Inouye K (1964) Syntheses of peptides related to the N-terminal structure of corticotropin. III. Synthesis of L-histidyl-l-phenylalanyl-l-arginyl-l-tryptophan, the smallest peptide exhibiting the melanocyte-stimulating and the lipolytic activities. Bull Chem Soc Japan 37:1465–1471
Pallavicini M, Bolchi C, Fumagalli L, Piccolo O, Valoti E (2010) A highly efficient method for the alpha, beta-dehydrogenation of alpha-amino esters and alpha-amino-beta diesters. Tetrahedron Lett 51:5540–5542
Prat D, Pardigon O, Flemming HW, Letestu S, Ducandas V, Isnard P, Guntrum E, Senac T, Ruisseau S, Cruciani P et al (2013) Sanofi’s solvent selection guide: a step toward more sustainable processes. Org Process Res Dev 17:1517–1525
Prat D, Wells A, Hayler J, Sneddon H, McElroy RC, Abou-Shehada S, Dunn PJ (2016) CHEM21 selection guide of classical- and less classical-solvents. Green Chem 18:288–296
Smith GG, Sivakua T (1983) Mechanism of the racemization of amino acids. Kinetics of racemization of arylglycines. J Org Chem 48:627–634
Straniero V, Pallavicini M, Chiodini G, Ruggeri P, Fumagalli L, Bolchi C, Corsini A, Ferri N, Ricci C, Valoti E (2014) Farnesyl transferase inhibitors: CAAX mimetics based on different biaryl scaffolds. Bioorg Med Chem Lett 24:2924–2927
Taglang C, Martinez-Prieto LM, del Rosal I, Maron L, Poteau R, Philippot K, Chaudret B, Perato S, Lone AS, Puente C, Dugave C, Rousseau B, Pieters G (2015) Enantiospecific C–H activation using ruthenium nanocatalysts. Angew Chem Int Ed 54:10474–10477
Tekkam S, Johnson JL, Jonnalagadda SC, Mereddy VR (2013) Concise synthesis of α-methylene-β-hydroxy-γ-carboxy-γ-lactams. J Heterocyclic Chem 50:955–958
Unger N, Holzgrabe U (2018) Stability and assessment of amino acids in parenteral nutrition solutions. J Pharm Biomed Anal 147:125–139
Verdié P, Subra G, Averland-Petit M-C, Amblard M, Martinez J (2008) Solid-phase synthesis of 4-methylcarbpxy-1,4-benzodiazepine-2,5-diones. J Comb Chem 10:869–874
Wilchek M, Patchornik A (1962) The synthesis of tryptophan peptides. J Org Chem 28:1875
Zanna N, Milli L, Del Secco B, Tomasini C (2016) Factors affecting the stabilization of polyproline II helices in a hydrophobic environment. Org Lett 18:1662–1665
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: P. Meffre.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bolchi, C., Bavo, F., Regazzoni, L. et al. Preparation of enantiopure methionine, arginine, tryptophan, and proline benzyl esters in green ethers by Fischer–Speier reaction. Amino Acids 50, 1261–1268 (2018). https://doi.org/10.1007/s00726-018-2599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2599-2