Abstract
The aim was to investigate whether there was an association between amino acid (AA) intake and physical fitness and if so, to assess whether this association was independent of carbohydrates intake. European adolescents (n = 1481, 12.5–17.5 years) were measured. Intake was assessed via two non-consecutive 24-h dietary recalls. Lower and upper limbs muscular fitness was assessed by standing long jump and handgrip strength tests, respectively. Cardiorespiratory fitness was assessed by the 20-m shuttle run test. Physical activity was objectively measured. Socioeconomic status was obtained via questionnaires. Lower limbs muscular fitness seems to be positively associated with tryptophan, histidine and methionine intake in boys, regardless of centre, age, socioeconomic status, physical activity and total energy intake (model 1). However, these associations disappeared once carbohydrates intake was controlled for (model 2). In girls, only proline intake seems to be positively associated with lower limbs muscular fitness (model 2) while cardiorespiratory fitness seems to be positively associated with leucine (model 1) and proline intake (models 1 and 2). None of the observed significant associations remained significant once multiple testing was controlled for. In conclusion, we failed to detect any associations between any of the evaluated AAs and physical fitness after taking into account the effect of multiple testing.
Similar content being viewed by others
Introduction
Physical fitness has been associated with health-related outcomes in children and adolescents (Ortega et al. 2008b). Results from longitudinal studies indicate that a higher level of physical fitness in young population is associated with a healthier cardiovascular profile when they become adults (Ruiz et al. 2009).
Although physical fitness is in part genetically determined, it is also influenced by environmental factors, mainly physical activity, and it is not well understood how it is associated with nutrition. Few studies have examined the association between dietary intake and physical fitness in adults; overall, they conclude that a healthy diet is positively associated with cardiorespiratory fitness (CRF) levels (Brodney et al. 2001; Haraldsdottir and Andersen 1994; Shikany et al. 2013). We previously observed a higher intake of dairy products and bread/cereals and a lower consumption of sweetened beverages in adolescents with high CRF (Cuenca-Garcia et al. 2012). However, specific macronutrients need still to be studied in detail due to their potential physiological interaction with physical fitness. Dietary protein and, more specifically, intakes of specific amino acids (AA) contribute to the growth, repair and maintenance of muscle cells and thus in the physical performance (Phillips 2012). In this line, dietary AA supplementation seems to be associated with muscle growth and athletic performance (Wu 2009). Branched-chain AA (BCAA), among others, help maintaining muscle tissue and are required when doing physical exercise (Matsumoto et al. 2009; Shimomura et al. 2004). However, no evidence exists yet about the specific role that AA might play on physical fitness. We hypothesize that higher intakes of AA might be associated with higher levels of muscular fitness and CRF, due to their physiological effects on muscle cells during and after exercise. In addition, dietary carbohydrates are one of the main energy sources for prolonged and low-intensity physical activities, and short, high-intensity exercises (Correia-Oliveira et al. 2013). Therefore, they have to be considered when examining the relationship between AA intake and physical fitness.
To our knowledge none has examined yet the association between the intake of a large number of AA and muscular fitness and CRF among adolescents. Since physical fitness has been associated with health outcomes, investigating whether dietary AA are positively related to muscular fitness and CRF is of both clinical and public health relevance. The purpose of this study is to investigate whether there is an association between AA intake and physical fitness in European adolescents and if so, to assess whether this association is independent of carbohydrates intake.
Methods
The current study is based on data derived from the Healthy Lifestyle in Europe by Nutrition in Adolescence cross-sectional study (HELENA-CSS) in which 3528 boys and girls aged 12.5–17.5 years had valid data for gender and body mass index (BMI). A subsample of 1481 adolescents (51.6% girls) were included in this report based on the following inclusion criteria: valid data on gender, BMI, AA intake, muscular fitness, CRF, physical activity (PA) and two 24-hour dietary recalls (24-HDR). Adolescents from the entire HELENA cohort were significantly older, weighed more and had higher mean BMI (all p < 0.05) (data not shown) than those included in this study.
The study was approved by the Research Ethics Committees of each city involved and was performed following the ethical guidelines of the Declaration of Helsinki, 1964 (revision of Edinburgh 2000). A written informed consent form was obtained from the adolescents and their parents.
A physical examination was performed with participants barefoot and wearing underwear. Briefly, body weight was measured with an electronic scale (Type SECA 861; range 0.05–130 kg; precision 0.05 kg). Height was measured in the Frankfurt plane with a telescopic height measuring instrument (Type SECA 225; range 60–200 cm; precision 1 mm). BMI was calculated as body weight (kg) divided by the height squared (m2).
Physical fitness was measured using tests that have been shown to be reliable in young people (Ortega et al. 2008a). The handgrip test (kg) was used to assess upper limbs muscular fitness. The ratio between handgrip and body mass was used in this report due to their significant correlation (r = 0.67 and r = 0.48 in boys and girls, respectively; all p < 0.001). In addition, previous studies have observed that weight status plays a positive role in handgrip performance in adolescents (Artero et al. 2010). The standing long jump test (cm) was used to assess lower limbs muscular fitness. The ratio between standing long jump and height was used in this report. The 20-m shuttle run test (stage) was used to assess CRF and VO2max (ml/kg/min) was estimated (Leger et al. 1988). As results from this study indicated that VO2max (ml/kg/min) penalized heavier adolescents, VO2max was expressed relative to body mass as a power function ratio standard (Tolfrey et al. 2006), with body mass raised to the power 0.77 (ml/kg−0.77/min). The suitability of an exponent of 0.77 was determined by log–log transformations and subsequent linear regression on the raw data.
Dietary intake was assessed by the HELENA-DIAT (Dietary Assessment Tool), a self-administered computer-based tool shown to accurately assess dietary information of European adolescents (Vereecken et al. 2008). Two non-consecutive 24-HDR within a time span of 2 weeks were obtained from each participant during school time and assisted by fieldworkers. The German Food Code and Nutrition Data Base (Bundeslebensmittelschlüssel, BLS Version II.3.1) (Dehne et al. 1999) was used to calculate energy and nutrient intakes. The usual food and nutrients intake was estimated by the Multiple Source Method which takes into account the within-person variability of the dietary data (Harttig et al. 2011). Energy intake was estimated in kilocalories per day (kcal/d), carbohydrate, protein and fat intake in grams per day (g/d) and grams per kilograms of body weight and per day (g/kg/d) and AA intake in milligrams per day (mg/d).
The Family Affluence Scale (FAS) is a valid socioeconomic status index in young people and has been previously used in large epidemiologic studies. It is based on the concept of material conditions in the family related to family expenditure and consumption (affluence) (Currie et al. 1997). The answers from all the questions were summed (range 0–8) and then grouped into three levels: low (0–2), medium (3–5), and high (6–8).
Uni-axial accelerometers (Actigraph MTI, model GT1M, Manufacturing Technology Inc., Fort Walton Beach, FL, USA) were used to objectively measure PA. At least three days of recording, with a minimum of 8 h registration per day, was set as an inclusion criterion. The time sampling interval (epoch) was set at 15 s. Average PA, expressed as mean counts per minute was used as a measure of overall PA.
Analyses were performed using the Statistical Package for Social Sciences software (SPSS, version 21.0 for WINDOWS; SPSS, Chicago, IL, USA) and values of p < 0.05 were considered statistically significant. After log-transformation of AA intakes, all variables showed a normal distribution. Since interactions between sex and the studied variables were observed (p < 0.05), results are given separately by sex. Descriptive data were assessed by one-way ANOVA for normally distributed variables and by Mann–Whitney U test for non-normally distributed variables. In case of categorical variables, the Chi-squared test was applied. Pearson correlation coefficients were calculated to analyse the association between total carbohydrate intake (g/day) and physical fitness. The association between AA intakes (independent variables) and fitness tests (dependent variables) was examined by multilevel linear regression analysis. Study centre was included as random intercept. Age, FAS, average PA and total daily energy intake (kcal/day) were entered as covariates in model 1. Model 2 included covariates from model 1 plus total carbohydrate intake (g/d). Significant associations (p < 0.05) found in the multilevel linear regression analyses (models 1 and/or 2) were examined more in depth by analyses of covariance (ANCOVA). Tertiles of AA intakes were entered as fixed factor, physical fitness variables were entered as dependent variables and study centre, age, FAS, average PA, total daily energy intake (kcal/day) and carbohydrate intake (g/day) were entered as covariates.
Results
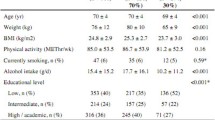
Descriptive data are provided in Table 1. Except for mean age and BMI all other analysed traits differed by gender.
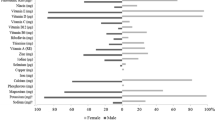
Dietary characteristics of the participants are provided in Table 2. Mann–Whitney U test showed that except for total carbohydrate intake (% energy), total protein intake (% energy) and total fat intake (% energy) all other analysed traits differed by gender. In addition, total carbohydrate intake (g/day) was significantly correlated with the physical fitness variables included in this study (r ranged from 0.302 to 0.361; all p < 0.001; Fig. 1).
Multilevel linear regression analyses of the associations between specific AA intakes and physical fitness are displayed in Tables 3, 4 and 5. In boys, tryptophan, histidine and methionine were the only to be (positively) associated with lower limbs muscular fitness (Table 3) in model 1. However, these associations disappeared after adjusting for total carbohydrates intake (g/day). In girls, proline was the only AA positively associated with lower limbs muscular fitness (model 2). Also in girls, leucine (model 1) and proline (models 1 and 2) were positively associated with CRF (Table 4) while no association was found between AA intakes and CRF in boys. No significant associations were found among any of the AA and upper limbs muscular fitness neither in boys nor in girls (Table 5). In addition, analyses were re-run by replacing the confounding variable total carbohydrate intake (g/day) by total carbohydrate intake (% of energy) and the results did not vary (data not shown). However, none of the observed associations were significant after controlling for multiple testing (0.05/number of tests = 0.05/18 = 0.003).
ANCOVA analyses of the associations between AA intake and physical fitness are shown in Fig. 2. Results showed that there were no significant differences between tertiles of AA intake and physical fitness neither in boys and girls.
Differences in fitness according to AA intake (tertiles) in adolescents adjusted for study centre, age, family affluence scale, average physical activity, total daily energy intake and carbohydrate intake (g/day). Boys’ tertiles in mg/day: tryptophan (<1052.2, 1052.2–1289.8, >1289.8); histidine (<2483.5, 2483.5–3113.7, >3113.7); methionine (<1989.5, 1989.5–2496.1, >2496.1); leucine (<7154.7, 7154.7–8835.8, >8835.8); proline (<6538, 6538–8098.7, >8098.7). Girls’ tertiles in mg/day: tryptophan (<804.9, 804.9–987.6, >987.6); histidine (<1898.4; 1898.4–2367.5, >2367.5); methionine (<1509.6, 1509.6–1898.4, >1898.4); leucine (<5435.1, 5435.1–6766.1.8, >6766.1); proline (<5000.3, 5000.3–6063.6, >6063.6). Tertiles were calculated with raw data to be more meaningful although differences were examined with the log-transformed variables. All p > 0.05. CRF cardiorespiratory fitness
Discussion
To the best of our knowledge, this is the first study analysing the relationship between a large number of dietary AA and physical fitness in adolescents. AA intake was measured by means of two self-administered, computer-assisted, non-consecutive 24-HDR which have been shown to be appropriate in collecting detailed dietary data in adolescents (Vereecken et al. 2008). The physical fitness tests included in this study have been shown to be reliable in young people (Ortega et al. 2008a). By definition, essential AA (EAA) cannot be synthesized de novo by the organism and, therefore, they must be supplied in the diet. Pancreatic enzymes convert the diet-ingested proteins into AA in the lumen of the small intestine (Pasini et al. 2004). AA are absorbed from the small intestine and enter the portal vein for protein synthesis in skeletal muscle and other tissues (Wu 2016). Skeletal muscle has an active role in AA metabolism by synthesizing alanine and glutamine from circulating BCAA (Wu 2009). Furthermore, the skeletal muscle plays a key role during exercise and stores the biggest amount of AA in the body. It regulates the movement of AA (incorporate or release AA) according to the needs of the organism and the balance between catabolic and anabolic state. For example, when catabolism is prevalent (e.g. during exercise), AA are released by the skeletal muscle to subsequently be converted into glucose by the liver to help in the functioning of the glucose-dependent organs (Carubelli et al. 2015).
In our study, lower limbs muscular strength seems to be positively associated with some EAA such as tryptophan, histidine and methionine after controlling for centre, age, FAS, PA and total energy intake in boys. It is well known that an increase in muscle mass can be achieved via nutritional supplementation. Indeed, it has been suggested that dietary supplementation with one or a mixture of functional AA, such as leucine, proline and tryptophan, among others, may be beneficial for optimizing efficiency of metabolic transformations to enhance muscle growth and athletic performance (Wu 2009). However, increases in muscle mass do not always accompany increases in muscle strength. Previous studies in older women observed that EAA supplementation increased muscle mass but not muscle strength (Dillon et al. 2009; Kim et al. 2012). Interestingly, muscle strength only improved when exercise and AA supplementation were combined (Kim et al. 2012). In the current study with adolescents, PA was controlled for and significant associations only disappeared after adjusting for total carbohydrate intake, suggesting that a specific macronutrient such as carbohydrate has a stronger confounding role than the one exerted by PA or total energy intake in these associations. All significant reported associations were weak and disappeared after controlling for multiple testing.
In our study, CRF (VO2max) seems to be positively associated with an EAA such as leucine and a BCAA such as proline after controlling for centre, age, FAS, PA and total energy intake in girls. Once carbohydrate intake was considered as a covariate, the association between leucine and VO2max disappeared. BCAAs account for 35% of the EAA in muscle proteins and 40% of the preformed amino acids required by mammals (Shimomura et al. 2004). BCAA help maintaining muscle tissue and are required during times of physical stress and intense exercise, characteristic of a VO2max test. BCAA ingestion immediately before an incremental load exercise test following chronic (6-d) BCAA supplementation significantly increased VO2max (Matsumoto et al. 2009) in young adults. BCAA excretion (leucine, isoleucine, and valine) was significantly lower in healthy adults with high fitness, as indicated by lower urinary levels of AA (Morris et al. 2013). As a response to exercise, AA biosynthesis and protein breakdown in skeletal muscle may increase (Rennie and Tipton 2000), which could possibly increase the systemic pool of AA. Therefore, exercise increases energy expenditure and as a consequence promotes oxidation of BCAA (Shimomura et al. 2004). Previous studies have also shown that EAA supplementation improves CRF in ambulatory chronic heart failure patients (Aquilani et al. 2008; Scognamiglio et al. 2008), with this being explained by improved muscle aerobic metabolism, prevalence of muscle anabolic processes and reduction of insulin resistance. Our results from a sample of healthy adolescents suggest that AA intake may have a positive influence on physical fitness because of the AA’s removal by active skeletal muscle during exercise and the increase in oxidation as exercise progresses. However, these findings should be interpreted cautiously as observed associations were weak and might be simply due to chance. In fact, no associations are found once statistical significance is controlled for multiple testing.
Model 2 was adjusted for carbohydrates intake to account for any potential confounding role that it might play in the association between AA and physical fitness. Dietary carbohydrates are one of the main fuels for sport activities, and their relevance for optimal sport performance is undisputed among experts, improving performance in both prolonged, low-intensity and short, high-intensity exercises (Correia-Oliveira et al. 2013). In general, there is a consensus claiming an ergogenic effect of carbohydrates ingested just before or during a performance bout (Colombani et al. 2013). Carbohydrate feeding prior to exercise provides additional supplies for oxidation, resulting in increased muscle glucose uptake and reduced liver glucose output during exercise, and enhanced blood glucose availability which may preserve muscle glycogen stores (Jamurtas et al. 2011). In addition, higher carbohydrates intake is accompanied of higher insulin secretion, which is a determining factor of AA incorporation into muscle cells and proteins (Gower and Goss 2015). The fact that significant associations between AA intake and lower limbs muscular strength and CRF disappeared after controlling for carbohydrates intake could reflect that those adolescents that performed better in both physical fitness tests might have had higher carbohydrates intake compared to those who did worse. It is likely that these adolescents had also higher daily PA levels, explaining their higher carbohydrates consumption, as main energy source, which may occur along with an increased intake of proteins, as AA precursors, to enhance muscle development.
Despite the lack of significant associations in this sample of European healthy adolescents, it is noteworthy to highlight the key role of protein nutrition on health. Adolescence is a period of rapid development which entails increased tissue generation and protein gain; therefore, protein requirements are increased and adequate protein intake is crucial for optimal growth and, in the long term, for healthy ageing (Wu 2016). Although previous research has shown a decrease in PA levels in adolescents (Ruiz et al. 2011), mainly among girls, adolescence is still characterized for high levels of PA performance as compared to other periods of life. While sedentary behaviour seems to exert a detrimental effect on skeletal muscle, dietary protein and moderate exercise have synergistic effects on the protein synthesis of skeletal muscle (Wu 2016). Furthermore, evidence shows that PA combined with an increased intake of high-quality proteins may represent an effective strategy to enhance fat loss while preserving muscle mass (Wu 2016). Protein is a major component of bones and plays a key role in skeletal health to reduce risk for osteopenia and osteoporosis by regulating the efficiency of the absorption of dietary minerals and bone mineralization; high protein intake, however, can contribute to bone loss with the stimulation of calcium urinary excretion (Bonjour 2011). In this regard, dietary protein intake has also been linked to negative health outcomes. Excessive protein intake may cause intestinal, hepatic, renal and/or cardiovascular dysfunction in healthy people (Pedersen et al. 2013) and large animal protein intakes could be associated with an increase in risks of cancer and diabetes (Levine et al. 2014; van Nielen et al. 2015).
The cross-sectional design of this study does not allow for causality interpretations. Increasing the number of recording days would have been desirable to compensate for day-to-day variability in the 24HDR; however, dietary data were corrected for between- and within-person variability to partially mitigate this limitation and adolescents’ usual intakes were calculated using the Multiple Source Method to obtain more accurate intake estimates (Harttig et al. 2011). The most exhaustive food composition table available in Europe was used to compute nutrient intakes (Dehne et al. 1999). Although variability in nutrient content across countries is always present, the applied food composition table was considered a good alternative to national food composition tables (Julian-Almarcegui et al. 2016). Nevertheless, dietary assessment methods are subject to measurement error and it cannot be precluded certain degree of inaccuracy when computing nutrient intakes, including amino acids intake. Despite the aforementioned limitations, this is the first study reporting the association between different physical fitness components and a large number of AA in adolescents. The fitness tests used in the present report have shown a good criterion-related validity in adolescents. Bonferroni correction was applied to counteract for the multiple testing problem, which is considered the most conservative method to control the familywise error rate.
Conclusions
We failed to detect any associations in this sample of healthy European adolescents between any of the evaluated AAs and physical fitness after taking into account the effect of multiple testing.
References
Aquilani R, Viglio S, Iadarola P, Opasich C, Testa A, Dioguardi FS, Pasini E (2008) Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol 101:104E–110E. doi:10.1016/j.amjcard.2008.03.008
Artero EG et al (2010) Health-related fitness in adolescents: underweight, and not only overweight, as an influencing factor. AVENA Study Scand J Med Sci Sports 20:418–427. doi:10.1111/j.1600-0838.2009.00959.x
Bonjour JP (2011) Protein intake and bone health. Int J Vitam Nutr Res 81:134–142. doi:10.1024/0300-9831/a000063
Brodney S, McPherson RS, Carpenter RS, Welten D, Blair SN (2001) Nutrient intake of physically fit and unfit men and women. Med Sci Sports Exerc 33:459–467
Carubelli V, Castrini AI, Lazzarini V, Gheorghiade M, Metra M, Lombardi C (2015) Amino acids and derivatives, a new treatment of chronic heart failure? Heart Fail Rev 20:39–51. doi:10.1007/s10741-014-9436-9
Colombani PC, Mannhart C, Mettler S (2013) Carbohydrates and exercise performance in non-fasted athletes: a systematic review of studies mimicking real-life. Nutr J 12:16. doi:10.1186/1475-2891-12-16
Correia-Oliveira CR, Bertuzzi R, Dal’Molin Kiss MA, Lima-Silva AE (2013) Strategies of dietary carbohydrate manipulation and their effects on performance in cycling time trials. Sports Med 43:707–719. doi:10.1007/s40279-013-0054-9
Cuenca-Garcia M et al (2012) Cardiorespiratory fitness and dietary intake in European adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 107:1850–1859. doi:10.1017/S0007114511005149
Currie CE, Elton RA, Todd J, Platt S (1997) Indicators of socioeconomic status for adolescents: the WHO Health Behaviour in School-aged Children Survey. Health Educ Res 12:385–397
Dehne LI, Klemm C, Henseler G, Hermann-Kunz E (1999) The German Food Code and Nutrient Data Base (BLS II.2). Eur J Epidemiol 15:355–359
Dillon EL et al (2009) Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab 94:1630–1637. doi:10.1210/jc.2008-1564
Gower BA, Goss AM (2015) A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. J Nutr 145:177S–183S. doi:10.3945/jn.114.195065jn.114.195065
Haraldsdottir J, Andersen LB (1994) Dietary factors related to fitness in young men and women. Prev Med 23:490–497
Harttig U, Haubrock J, Knuppel S, Boeing H, Consortium E (2011) The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr 65(suppl 1):S87–S91. doi:10.1038/ejcn.2011.92
Jamurtas AZ et al (2011) The effects of low and high glycemic index foods on exercise performance and beta-endorphin responses. J Int Soc Sports Nutr 8:15. doi:10.1186/1550-2783-8-15
Julian-Almarcegui C et al (2016) Comparison of different approaches to calculate nutrient intakes based upon 24-h recall data derived from a multicenter study in European adolescents. Eur J Nutr 55:537–545. doi:10.1007/s00394-015-0870-9
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M (2012) Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 60:16–23. doi:10.1111/j.1532-5415.2011.03776.x
Leger LA, Mercier D, Gadoury C, Lambert J (1988) The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci 6:93–101
Levine ME et al (2014) Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19:407–417. doi:10.1016/j.cmet.2014.02.006
Matsumoto K, Koba T, Hamada K, Tsujimoto H, Mitsuzono R (2009) Branched-chain amino acid supplementation increases the lactate threshold during an incremental exercise test in trained individuals. J Nutr Sci Vitaminol (Tokyo) 55:52–58
Morris C et al (2013) The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol Nutr Food Res 57:1246–1254. doi:10.1002/mnfr.201200629
Ortega FB et al (2008a) Reliability of health-related physical fitness tests in European adolescents. HELENA Study Int J Obes (Lond) 32(Suppl 5):S49–S57
Ortega FB, Ruiz JR, Castillo MJ, Sjostrom M (2008b) Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond) 32:1–11
Pasini E, Aquilani R, Dioguardi FS (2004) Amino acids: chemistry and metabolism in normal and hypercatabolic states. Am J Cardiol 93:3A–5A. doi:10.1016/j.amjcard.2003.11.001
Pedersen AN, Kondrup J, Borsheim E (2013) Health effects of protein intake in healthy adults: a systematic literature review Food. Nutr Res. doi:10.3402/fnr.v57i0.21245
Phillips SM (2012) Dietary protein requirements and adaptive advantages in athletes. Br J Nutr 108(suppl 2):S158–S167. doi:10.1017/S0007114512002516
Rennie MJ, Tipton KD (2000) Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr 20:457–483. doi:10.1146/annurev.nutr.20.1.457
Ruiz JR, Castro-Pinero J, Artero EG, Ortega FB, Sjostrom M, Suni J, Castillo MJ (2009) Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med 43:909–923. doi:10.1136/bjsm.2008.056499
Ruiz JR et al (2011) Objectively measured physical activity and sedentary time in European Adolescents: the HELENA Study. Am J Epidemiol 174:173–184
Scognamiglio R, Testa A, Aquilani R, Dioguardi FS, Pasini E (2008) Impairment in walking capacity and myocardial function in the elderly: is there a role for nonpharmacologic therapy with nutritional amino acid supplements? Am J Cardiol 101:78E–81E. doi:10.1016/j.amjcard.2008.03.005
Shikany JM, Jacobs DR Jr, Lewis CE, Steffen LM, Sternfeld B, Carnethon MR, Richman JS (2013) Associations between food groups, dietary patterns, and cardiorespiratory fitness in the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr 98:1402–1409. doi:10.3945/ajcn.113.058826
Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA (2004) Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr 134:1583S–1587S
Tolfrey K, Barker A, Thom JM, Morse CI, Narici MV, Batterham AM (2006) Scaling of maximal oxygen uptake by lower leg muscle volume in boys and men. J Appl Physiol 100(1985):1851–1856. doi:10.1152/japplphysiol.01213.2005
van Nielen M et al (2015) Erratum. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care 38:1854–1862. doi:10.2337/dc15-er10b (Diabetes Care 2014;37)
Vereecken CA et al (2008) Development and evaluation of a self-administered computerized 24-h dietary recall method for adolescents in Europe. Int J Obes (Lond) 32(suppl 5):S26–S34
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17. doi:10.1007/s00726-009-0269-0
Wu G (2016) Dietary protein intake and human health Food Funct 7:1251–1265. doi:10.1039/c5fo01530h
Acknowledgements
The authors gratefully acknowledge all participating children and adolescents, and their parents and teachers, for their collaboration. The HELENA study took place with the financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT: 2005-007034). Nevertheless, the content of this paper reflects the authors’ views alone, and the European Community is not liable for any use that may be made of the information contained herein. European Commission had no role in the design, analysis or writing of this article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gracia-Marco, L., Bel-Serrat, S., Cuenca-Garcia, M. et al. Amino acids intake and physical fitness among adolescents. Amino Acids 49, 1041–1052 (2017). https://doi.org/10.1007/s00726-017-2393-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2393-6