Abstract
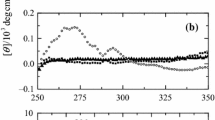
We analyzed the structure of horseradish peroxidase (HRP) under denaturing conditions of 9 M urea or 6 M guanidine hydrochloride (GdnHCl). Far-UV circular dichroism (CD) spectra indicated the existence of native-like secondary structure of holo-HRP in 9 M urea. In addition, slight changes in near-UV and Soret region CD spectra of holo-HRP in 9 M urea suggest that the tertiary structure of holo-HRP and the binding of heme remain partially intact in this condition. A transition in the thermal unfolding transition curve of holo-HRP in 9 M urea indicated the existence of a considerable amount of secondary structure. However, no secondary structure, tertiary structure, or interaction between heme and HRP were observed in holo-HRP in 6 M GdnHCl. Small-angle X-ray scattering indicated that although distal and proximal domains of holo-HRP in 9 M urea might be partially unfolded, the central region that contains the heme might maintain its tertiary structure. Our results suggest that retention of the heme is essential for maintenance of the structure of HRP under highly denaturing conditions.









Similar content being viewed by others
References
Akita M, Tsutsumi D, Kobayashi M, Kise H (2001) Structural change and catalytic activity of horseradish peroxidase in oxidative polymerization of phenol. Biosci Biotechnol Biochem 65(7):1581–1588. doi:10.1271/bbb.65.1581
Azevedo AM, Martins VC, Prazeres DM, Vojinovic V, Cabral JM, Fonseca LP (2003) Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev 9:199–247
Carvalho AS, Melo EP, Ferreira BS, Neves-Petersen MT, Petersen SB, Aires-Barros MR (2003) Heme and pH-dependent stability of an anionic horseradish peroxidase. Arch Biochem Biophys 415(2):257–267
Cha HJ, Jang DS, Kim YG, Hong BH, Woo JS, Kim KT, Choi KY (2013) Rescue of deleterious mutations by the compensatory Y30F mutation in ketosteroid isomerase. Mol Cells 36(1):39–46. doi:10.1007/s10059-013-0013-1
Cha HJ, Jang DS, Jin KS, Lee HJ, Hong BH, Kim ES, Kim J, Lee HC, Choi KY, Lee M (2014) Three-dimensional structures of a wild-type ketosteroid isomerase and its mutant in solution. Sci Adv Mater 6(11):2325–2333
Chattopadhyay K, Mazumdar S (2000) Structural and conformational stability of horseradish peroxidase: effect of temperature and pH. Biochemistry 39(1):263–270
Cockle SA, Epand RM, Moscarello MA (1978) Resistance of lipophilin, a hydrophobic myelin protein, to denaturation by urea and guanidinium salts. J Biol Chem 253(22):8019–8026
Feng JY, Liu JZ, Ji LN (2008) Thermostability, solvent tolerance, catalytic activity and conformation of cofactor modified horseradish peroxidase. Biochimie 90(9):1337–1346. doi:10.1016/j.biochi.2008.03.010
Franke D, Svergun DI (2009) DAMMIF, a program for rapid ab initio shape determination in small-angle scattering. J Appl Crystallogr 42:342–346
Gajhede M, Schuller DJ, Henriksen A, Smith AT, Poulos TL (1997) Crystal structure of horseradish peroxidase C at 2.15 A resolution. Nat Struct Biol 4(12):1032–1038
Glatter O, Kratky O (1982) Small angle X-ray scattering. Academic Press, London
Greenfield NJ (2006) Using circular dichroism collected as a function of temperature to determine the thermodynamics of protein unfolding and binding interactions. Nat Protoc 1(6):2527–2535. doi:10.1038/nprot.2006.204
Haque E, Debnath D, Basak S, Chakrabarti A (1999) Structural changes of horseradish peroxidase in presence of low concentrations of urea. Eur J Biochem 259(1–2):269–274
Hargrove MS, Olson JS (1996) The stability of holomyoglobin is determined by heme affinity. Biochemistry 35(35):11310–11318. doi:10.1021/bi9603736
Howes BD, Feis A, Raimondi L, Indiani C, Smulevich G (2001) The critical role of the proximal calcium ion in the structural properties of horseradish peroxidase. J Biol Chem 276(44):40704–40711. doi:10.1074/jbc.M107489200
Hsu MC, Woody RW (1971) The origin of the heme Cotton effects in myoglobin and hemoglobin. J Am Chem Soc 93(14):3515–3525
Jang DS, Lee HJ, Lee B, Hong BH, Cha HJ, Yoon J, Lim K, Yoon YJ, Kim J, Ree M, Lee HC, Choi KY (2006) Detection of an intermediate during the unfolding process of the dimeric ketosteroid isomerase. FEBS Lett 580(17):4166–4171. doi:10.1016/j.febslet.2006.06.069
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751(2):119–139. doi:10.1016/j.bbapap.2005.06.005
Kim ES, Jang DS, Yang SY, Lee MN, Jin KS, Cha HJ, Kim JK, Sung YC, Choi KY (2013) Controlled release of human growth hormone fused with a human hybrid Fc fragment through a nanoporous polymer membrane. Nanoscale 5(10):4262–4269. doi:10.1039/c3nr00474k
Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI (2003) PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr 36:1277–1282
Koua D, Cerutti L, Falquet L, Sigrist CJ, Theiler G, Hulo N, Dunand C (2009) PeroxiBase: a database with new tools for peroxidase family classification. Nucleic Acids Res 37(Database issue):D261–D266. doi:10.1093/nar/gkn680
Kozin MB, Svergun DI (2001) Automated matching of high- and low-resolution structural models. J Appl Crystallogr 34:33–41
Krainer FW, Glieder A (2015) An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Appl Microbiol Biotechnol 99(4):1611–1625. doi:10.1007/s00253-014-6346-7
Kundu J, Kar U, Gautam S, Karmakar S, Chowdhury PK (2015) Unusual effects of crowders on heme retention in myoglobin. FEBS Lett 589(24 Pt B):3807–3815. doi:10.1016/j.febslet.2015.11.015
Mao L, Luo S, Huang Q, Lu J (2013) Horseradish peroxidase inactivation: heme destruction and influence of polyethylene glycol. Sci Rep 3:3126. doi:10.1038/srep03126
Mogharrab N, Ghourchian H, Amininasab M (2007) Structural stabilization and functional improvement of horseradish peroxidase upon modification of accessible lysines: experiments and simulation. Biophys J 92(4):1192–1203. doi:10.1529/biophysj.106.092858
Monhemi H, Housaindokht MR, Moosavi-Movahedi AA, Bozorgmehr MR (2014) How a protein can remain stable in a solvent with high content of urea: insights from molecular dynamics simulation of Candida antarctica lipase B in urea: choline chloride deep eutectic solvent. Phys Chem Chem Phys 16(28):14882–14893. doi:10.1039/c4cp00503a
Myer YP (1968) Conformation of cytochromes. III. Effect of urea, temperature, extrinsic ligands, and pH variation on the conformation of horse heart ferricytochrome c. Biochemistry 7(2):765–776
Pappa HS, Cass AE (1993) A step towards understanding the folding mechanism of horseradish peroxidase. Tryptophan fluorescence and circular dichroism equilibrium studies. Eur J Biochem 212(1):227–235
Poulos TL (1993) Peroxidases. Curr Opin Biotechnol 4(4):484–489
Rambo RP, Tainer JA (2011) Characterizing flexible and intrinsically unstructured biological macromolecules by SAS using the Porod-Debye law. Biopolymers 95(8):559–571. doi:10.1002/bip.21638
Ryan BJ, O’Fagain C (2007) Arginine-to-lysine substitutions influence recombinant horseradish peroxidase stability and immobilisation effectiveness. BMC Biotechnol 7:86. doi:10.1186/1472-6750-7-86
Semenyuk AV, Svergun DI (1991) GNOM—a program package for small-angle scattering data processing. J Appl Crystallogr 24:537–540
Soh YM, Burmann F, Shin HC, Oda T, Jin KS, Toseland CP, Kim C, Lee H, Kim SJ, Kong MS, Durand-Diebold ML, Kim YG, Kim HM, Lee NK, Sato M, Oh BH, Gruber S (2015) Molecular basis for SMC rod formation and its dissolution upon DNA binding. Mol Cell 57(2):290–303. doi:10.1016/j.molcel.2014.11.023
Strickland EH (1968) Circular dichroism of horseradish peroxidase and its enzyme-substrate compounds. Biochim Biophys Acta 151(1):70–75
Strickland EH, Kay E, Shannon LM, Horwitz J (1968) Peroxidase isoenzymes from horseradish roots. 3. Circular dichroism of isoenzymes and apoisoenzymes. J Biol Chem 243(13):3560–3565
Svergun D, Barberato C, Koch MHJ (1995) CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Crystallogr 28:768–773
Tamura M, Asakura T, Yonetani T (1972) Heme-modification studies on horseradish peroxidase. Biochim Biophys Acta 268(2):292–304
Tanford C (1968) Protein denaturation. Adv Protein Chem 23:121–282
Uppal S, Salhotra S, Mukhi N, Zaidi FK, Seal M, Dey SG, Bhat R, Kundu S (2015) Significantly enhanced heme retention ability of myoglobin engineered to mimic the third covalent linkage by nonaxial histidine to heme (vinyl) in synechocystis hemoglobin. J Biol Chem 290(4):1979–1993. doi:10.1074/jbc.M114.603225
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65(3):249–259
Volkov VV, Svergun DI (2003) Uniqueness of ab initio shape determination in small-angle scattering. J Appl Crystallogr 36:860–864
Wagner M, Nicell JA (2002) Detoxification of phenolic solutions with horseradish peroxidase and hydrogen peroxide. Water Res 36(16):4041–4052
Wang C, Chen Z, Hong X, Ning F, Liu H, Zang J, Yan X, Kemp J, Musselman CA, Kutateladze TG, Zhao R, Jiang C, Zhang G (2014) The structural basis of urea-induced protein unfolding in beta-catenin. Acta Crystallogr D Biol Crystallogr 70(Pt 11):2840–2847. doi:10.1107/S1399004714018094
Won K, Kim YH, An ES, Lee YS, Song BK (2004) Horseradish peroxidase-catalyzed polymerization of cardanol in the presence of redox mediators. Biomacromolecules 5(1):1–4. doi:10.1021/bm034325u
Yonetani T (1967) Studies on cytochrome c peroxidase. X. Crystalline apo-and reconstituted holoenzymes. J Biol Chem 242(21):5008–5013
Yu H, Huang H (2014) Engineering proteins for thermostability through rigidifying flexible sites. Biotechnol Adv 32(2):308–315. doi:10.1016/j.biotechadv.2013.10.012
Zakharova GS, Uporov IV, Tishkov VI (2011) Horseradish peroxidase: modulation of properties by chemical modification of protein and heme. Biochemistry (Mosc) 76(13):1391–1401. doi:10.1134/S0006297911130037
Acknowledgements
This research was supported by a Grant from the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2014R1A2A2A01002931) and the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea (PJ01121601). This was also funded by the industrial research project from LG Chem.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: S. Dai.
H. J. Cha and D. S. Jang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cha, H.J., Jang, D.S., Jin, K.S. et al. Structural analyses combined with small-angle X-ray scattering reveals that the retention of heme is critical for maintaining the structure of horseradish peroxidase under denaturing conditions. Amino Acids 49, 715–723 (2017). https://doi.org/10.1007/s00726-016-2372-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2372-3