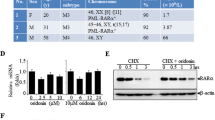
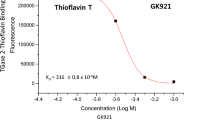
Abstract
Transglutaminase 2 (TG2) localizes to the nucleus and induces apoptosis through a crosslinking inactivation of Sp1 in JHH-7 cells treated with acyclic retinoid. We screened an inhibitor suppressing transamidase activity in the nucleus without affecting transamidase activity itself. Phenosafranin was found to inhibit nuclear localization of EGFP-tagged TG2 and dose-dependently reduce nuclear transamidase activity without affecting the activity in a tube. We concluded that phenosafranin was a novel TG2 inhibitor capable of suppressing its nuclear localization.


Similar content being viewed by others
Abbreviations
- TG:
-
Transglutaminase
- 5BAPA:
-
5-(Biotinamido) pentylamine
- ACR:
-
Acyclic retinoid
- DMEM:
-
Dulbecco’s modified Eagle medium
- PBS:
-
Phosphate-buffered saline
- NLS:
-
Nuclear localization signal
- NES:
-
Nuclear export signal
- NASH:
-
Non-alcoholic steatohepatitis
- ASH:
-
Alcoholic steatohepatitis
- Cys:
-
Cystamine
References
Ballestar E, Abad C, Franco L (1996) Core histones are glutaminyl substrates for tissue transglutaminase. J Biol Chem 271:18817–18824
Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K (2014) Transglutaminase regulation of cell function. Physiol Rev 94:383–417. doi:10.1152/physrev.00019.2013
Furutani Y, Kojima S (2015) Control of TG functions depending on their localization. In: Hitomi K, Kojima S, Fesus L (eds) Transglutaminases: multiple functional modifiers and targets for new drug discovery, 1st edn. Springer, Tokyo, pp 43–62. doi:10.1007/978-4-431-55825-5_2
Haddox MK, Russell DH (1981) Increased nuclear conjugated polyamines and transglutaminase during liver regeneration. Proc Natl Acad Sci USA 78:1712–1716
Iismaa SE, Mearns BM, Lorand L, Graham RM (2009) Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 89:991–1023. doi:10.1152/physrev.00044.2008
Karpuj MV, Garren H, Slunt H, Price DL, Gusella J, Becher MW, Steinman L (1999) Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington’s disease brain nuclei. Proc Natl Acad Sci USA 96:7388–7393
Kato N, Takahashi S, Nogawa T, Saito T, Osada H (2012) Construction of a microbial natural product library for chemical biology studies. Curr Opin Chem Biol 16:101–108. doi:10.1016/j.cbpa.2012.02.016
Keillor JW, Apperley KY, Akbar A (2015) Inhibitors of tissue transglutaminase. Trends Pharmacol Sci 36:32–40. doi:10.1016/j.tips.2014.10.014
Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, Kojima S (2012) Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol 227:1130–1137. doi:10.1002/jcp.22833
Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ (2006) Phosphorylation of histones by tissue transglutaminase. J Biol Chem 281:5532–5538. doi:10.1074/jbc.M506864200
Shrestha R, Tatsukawa H, Shrestha R, Ishibashi N, Matsuura T, Kagechika H, Kose S, Hitomi K, Imamoto N, Kojima S (2015) Molecular mechanism by which acyclic retinoid induces nuclear localization of transglutaminase 2 in human hepatocellular carcinoma cells. Cell Death Dis 6:e2002. doi:10.1038/cddis.2015.339
Tatsukawa H, Fukaya Y, Frampton G, Martinez-Fuentes A, Suzuki K, Kuo TF, Nagatsuma K, Shimokado K, Okuno M, Wu J, Iismaa S, Matsuura T, Tsukamoto H, Zern MA, Graham RM, Kojima S (2009) Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 136(1783–1795):e1710. doi:10.1053/j.gastro.2009.01.007
Tatsukawa H, Sano T, Fukaya Y, Ishibashi N, Watanabe M, Okuno M, Moriwaki H, Kojima S (2011) Dual induction of caspase 3- and transglutaminase-dependent apoptosis by acyclic retinoid in hepatocellular carcinoma cells. Mol Cancer 10:4. doi:10.1186/1476-4598-10-4
Tatsukawa H, Furutani Y, Hitomi K, Kojima S (2016) Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis 7:e2244. doi:10.1038/cddis.2016.150
Uchida T, Hiraga N, Imamura M, Tsuge M, Abe H, Hayes CN, Aikata H, Ishida Y, Tateno C, Yoshizato K, Ohdan H, Murakami K, Chayama K (2015) Human cytotoxic T lymphocyte-mediated acute liver failure and rescue by immunoglobulin in human hepatocyte transplant TK-NOG mice. J Virol 89:10087–10096. doi:10.1128/jvi.01126-15
Acknowledgments
We thank the former laboratory members, including Dr. Tatsukawa (currently Nagoya Univ), Ms. Fukaya-Tatsukawa, Ms. Yoshioka, and Ms. Imazawa for their efforts in an initial screening of this study. JHH-7 cells were kindly gifted by Dr. Matsuura (Jikei Univ School of Med). NPDepo chemical library was kindly gifted by Dr. Osada and Dr. Saito (RIKEN). The authors’ experimental work referred to in this report was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan KAKENHI Grant Number JP26102742 (to SK); The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16K09386 (to YF); Advanced Research Networks; and the Research on the Innovative Development and the Practical Application of New Drugs for Hepatitis B (Principal Investigator: SK) provided by the Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editors: S. Beninati, M. Piacentini, C. M. Bergamini.
Rights and permissions
About this article
Cite this article
Furutani, Y., Toguchi, M., Shrestha, R. et al. Phenosafranin inhibits nuclear localization of transglutaminase 2 without affecting its transamidase activity. Amino Acids 49, 483–488 (2017). https://doi.org/10.1007/s00726-016-2337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2337-6