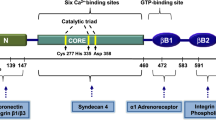
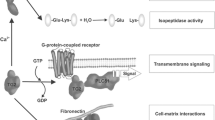
Abstract
Type 2 transglutaminase (TG2) has an important pathogenic role in celiac disease (CD), an inflammatory intestinal disease that is caused by the ingestion of gluten-containing cereals. Indeed, TG2 deamidates specific gliadin peptides, thus enhancing their immunogenicity. Moreover, the transamidating activity seems to provoke an autoimmune response, where TG2 is the main autoantigen. Many studies have highlighted a possible pathogenetic role of anti-TG2 antibodies, because they modulate TG2 enzymatic activity and they can interact with cell-surface TG2, triggering a wide range of intracellular responses. Autoantibodies also alter the uptake of the alpha-gliadin peptide 31–43 (p31–43), responsible of the innate immune response in CD, thus partially protecting cells from p31–43 damaging effects in an intestinal cell line. Here, we investigated whether anti-TG2 antibodies protect cells from p31–43-induced damage in a CD model consisting of primary dermal fibroblasts. We found that the antibodies specifically reduced the uptake of p31–43 by fibroblasts derived from healthy subjects but not in those derived from CD patients. Analyses of TG2 expression and enzymatic activity did not reveal any significant difference between fibroblasts from healthy and celiac subjects, suggesting that other features related to TG2 may be responsible of such different behaviors, e.g., trafficking or subcellular distribution. Our findings are in line with the concept that a “celiac cellular phenotype” exists and that TG2 may contribute to this phenotype. Moreover, they suggest that the autoimmune response to TG2, which alone may damage the celiac mucosa, also fails in its protective role in celiac cells.




Similar content being viewed by others
Abbreviations
- TG2:
-
Type 2 transglutaminase
- CD:
-
Celiac disease
- HLA:
-
Human leucocytes antigen
- p31–43:
-
Peptide 31–43
- p57–58:
-
Peptide 57–68
- PBS:
-
Phosphate-buffered saline
- PFA:
-
Paraformaldehyde
- M-β-CD:
-
Methyl-β-cyclodextrin
- liss-p31–43:
-
Lissamine-labeled p31–43
References
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148:825–838
Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C (2007) Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology 132(4):1245–1253
Barone MV, Troncone R, Auricchio S (2014) Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int J Mol Sci 15(11):20518–20537
Caja S, Mäki M, Kaukinen K, Lindfors K (2011) Antibodies in celiac disease: implications beyond diagnostics. Cell Mol Immunol 8(2):103–109
Caputo I, Barone MV, Martucciello S, Lepretti M, Esposito C (2009) Tissue transglutaminase in celiac disease: role of autoantibodies. Amino Acids 36(4):693–699
Caputo I, Barone MV, Lepretti M, Martucciello S, Nista I, Troncone R, Auricchio S, Sblattero D, Esposito C (2010) Celiac anti-tissue transglutaminase antibodies interfere with the uptake of alpha gliadin peptide 31-43 but not of peptide 57-68 by epithelial cells. Biochim Biophys Acta 1802(9):717–727
Caputo I, Secondo A, Lepretti M, Paolella G, Auricchio S, Barone MV, Esposito C (2012) Gliadin peptides induce tissue transglutaminase activation and ER-stress through Ca2+ mobilization in Caco-2 cells. PLoS One 7(9):e45209
Caputo I, Lepretti M, Secondo A, Martucciello S, Paolella G, Sblattero D, Barone MV, Esposito C (2013) Anti-tissue transglutaminase antibodies activate intracellular tissue transglutaminase by modulating cytosolic Ca2+ homeostasis. Amino Acids 44(1):251–260
Chen X, Hnida K, Graewert MA, Andersen JT, Iversen R, Tuukkanen A, Svergun D, Sollid LM (2015) Structural basis for antigen recognition by transglutaminase 2-specific autoantibodies in celiac disease. J Biol Chem 290(35):21365–21375
Di Niro R, Ziller F, Florian F, Crovella S, Stebel M, Bestagno M, Burrone O, Bradbury AR, Secco P, Marzari R, Sblattero D (2007) Construction of miniantibodies for the in vivo study of human autoimmune diseases in animal models. BMC Biotechnol 7:46
Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K (2014) Transglutaminase regulation of cell function. Physiol Rev 94(2):383–417
Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, Auricchio S, Troncone R (2003) Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol 98(8):1813–1820
Freshney RI (2011) Primary culture. In: Freshney RI (ed) Culture of animals cells: a manual of basic technique and specialized applications, edn. Wiley, New York, pp 163–186
Green PH (2005) The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 128:S74–S78
Halttunen T, Maki M (1999) Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 116:566–572
Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373:793–803
Iismaa SE, Mearns BM, Lorand L, Graham RM (2009) Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 89(3):991–1023
Im MJ, Russell MA, Feng JF (1997) Transglutaminase II: a new class of GTP-binding protein with new biological functions. Cell Signal 9:477–482
Iversen R, Mysling S, Hnida K, Jørgensen TJ, Sollid LM (2014) Activity-regulating structural changes and autoantibody epitopes in transglutaminase 2 assessed by hydrogen/deuterium exchange. Proc Natl Acad Sci U S A 111(48):17146–17151
Iversen R, Fleur du Pré M, Di Niro R, Sollid LM (2015) Igs as substrates for transglutaminase 2: implications for autoantibody production in celiac disease. J Immunol 195(11):5159–5168
Kalliokoski S, Sulic AM, Korponay-Szabó IR, Szondy Z, Frias R, Perez MA, Martucciello S, Roivainen A, Pelliniemi LJ, Esposito C, Griffin M, Sblattero D, Mäki M, Kaukinen K, Lindfors K, Caja S (2013) Celiac disease-specific TG2-targeted autoantibodies inhibit angiogenesis ex vivo and in vivo in mice by interfering with endothelial cell dynamics. PLoS One 8(6):e65887
Kalliokoski S, Caja S, Frias R, Laurila K, Koskinen O, Niemelä O, Mäki M, Kaukinen K, Korponay-Szabó IR, Lindfors K (2015) Injection of celiac disease patient sera or immunoglobulins to mice reproduces a condition mimicking early developing celiac disease. J Mol Med (Berl) 93(1):51–62
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4(2):140–156
Nanayakkara M, Kosova R, Lania G, Sarno M, Gaito A, Galatola M, Greco L, Cuomo M, Troncone R, Auricchio S, Auricchio R, Barone MV (2013a) A celiac cellular phenotype, with altered LPP sub-cellular distribution, is inducible in controls by the toxic gliadin peptide P31-43. PLoS One 8(11):e79763
Nanayakkara M, Lania G, Maglio M, Kosova R, Sarno M, Gaito A, Discepolo V, Troncone R, Auricchio S, Auricchio R, Barone MV (2013b) Enterocyte proliferation and signaling are constitutively altered in celiac disease. PLoS One 8(10):e76006
Paolella G, Caputo I, Marabotti A, Lepretti M, Salzano AM, Scaloni A, Vitale M, Zambrano N, Sblattero D, Esposito C (2013) Celiac anti-type 2 transglutaminase antibodies induce phosphoproteome modification in intestinal epithelial Caco-2 cells. PLoS One 8(12):e84403
Qiao SW, Bergseng E, Molberg O, Xia J, Fleckenstein B, Khosla C, Sollid LM (2004) Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol 173:1757–1762
Schuppan D, Dieterich W, Riecken EO (1998) Exposing gliadin as a tasty food for lymphocytes. Nat Med 4:666–667
Schuppan D, Junker Y, Barisani D (2009) Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137(6):1912–1933
Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297:2275–2279
Sollid LM (2000) Molecular basis of celiac disease. Annu Rev Immunol 18:53–81
Sollid LM, Jabri B (2011) Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol 23(6):732–738
Villanacci V, Not T, Sblattero D, Gaiotto T, Chirdo F, Galletti A, Bassotti G (2009) Mucosal tissue transglutaminase expression in celiac disease. J Cell Mol Med 13(2):334–340
Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A (2006) In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med 3(9):e358
Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM (2006) The role of tissue transglutaminase in cell-matrix interactions. Front Biosci 11:1057–1076
Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6(4):e19414
Acknowledgments
This work was supported by grant from Fondi di Ateneo per la Ricerca di Base of University of Salerno and from Associazione Italiana Celiachia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by “Fondi di Ateneo per la Ricerca di Base” (FARB), ORSA149715 and ORSA159291, and by “Associazione Italiana Celiachia” (AIC), FC 05_FC_2013.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol for this study was approved by the Ethical Committee of the University ‘‘Federico II’’, Naples, Italy (ethical approval: C.E. n. 230/05/E1).
Informed consent
All adult subjects provided written informed consent to use the biopsies in this study. Parents or tutors provided written informed consent for subjects under 18 years of age.
Additional information
Handling Editors: S. Beninati, M. Piacentini, C. M. Bergamini.
Rights and permissions
About this article
Cite this article
Paolella, G., Lepretti, M., Barone, M. et al. Celiac anti-type 2 transglutaminase antibodies induce differential effects in fibroblasts from celiac disease patients and from healthy subjects. Amino Acids 49, 541–550 (2017). https://doi.org/10.1007/s00726-016-2307-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2307-z