Abstract
Muscle glycogen availability can limit endurance exercise performance. We previously demonstrated 5 days of creatine (Cr) and carbohydrate (CHO) ingestion augmented post-exercise muscle glycogen storage compared to CHO feeding alone in healthy volunteers. Here, we aimed to characterise the time-course of this Cr-induced response under more stringent and controlled experimental conditions and identify potential mechanisms underpinning this phenomenon. Fourteen healthy, male volunteers cycled to exhaustion at 70 % VO2peak. Muscle biopsies were obtained at rest immediately post-exercise and after 1, 3 and 6 days of recovery, during which Cr or placebo supplements (20 g day−1) were ingested along with a prescribed high CHO diet (37.5 kcal kg body mass−1 day−1, >80 % calories CHO). Oral-glucose tolerance tests (oral-GTT) were performed pre-exercise and after 1, 3 and 6 days of Cr and placebo supplementation. Exercise depleted muscle glycogen content to the same extent in both treatment groups. Creatine supplementation increased muscle total-Cr, free-Cr and phosphocreatine (PCr) content above placebo following 1, 3 and 6 days of supplementation (all P < 0.05). Creatine supplementation also increased muscle glycogen content noticeably above placebo after 1 day of supplementation (P < 0.05), which was sustained thereafter. This study confirmed dietary Cr augments post-exercise muscle glycogen super-compensation, and demonstrates this occurred during the initial 24 h of post-exercise recovery (when muscle total-Cr had increased by <10 %). This marked response ensued without apparent treatment differences in muscle insulin sensitivity (oral-GTT, muscle GLUT4 mRNA), osmotic stress (muscle c-fos and HSP72 mRNA) or muscle cell volume (muscle water content) responses, such that another mechanism must be causative.
Similar content being viewed by others
Introduction
Muscle glycogen availability has long been cited as a principal determinant of endurance exercise performance, and its depletion corresponds with the development of muscle fatigue (Bergström and Hultman 1967; Bergström et al. 1967). Optimising liver and muscle glycogen storage is a goal of many athletes wishing to maximise performance, particularly those involved in disciplines requiring prolonged sub-maximal exertion (65–75 % VO2peak) or repeated bursts of high intensity exercise. The accepted method of maximising muscle glycogen content is by ‘carbohydrate (CHO) loading’, which classically involves the depletion of muscle glycogen reserves through prolonged sub-maximal exercise (>90 min), followed by the consumption of a high CHO diet (>70 % calories CHO) for several days (Bergström and Hultman 1966, 1967; Sherman and Costill 1984). The use of such methods has been shown to replenish muscle glycogen content to a habitual resting level within 24 h, and to super-compensate reserves by over 100 % within 48–72 h. As a result of this, improvements in subsequent endurance exercise performance (time to fatigue) of about 50 % can be expected (Bergström and Hultman 1967). This super-compensatory response is confined solely to the previously exercised (glycogen-depleted) muscle, and has been attributed to heightened muscle insulin sensitivity and a localised increase in glycogen synthase activity (Bergström and Hultman 1967, 1966; Jentjens and Jeukendrup 2003).
We have previously demonstrated that dietary Cr supplementation can augment post-exercise muscle glycogen storage during a conventional ‘CHO-loading’ regimen in healthy, young, male volunteers, and that this response is restricted to the previously exercised limb (Robinson et al. 1999; Sewell et al. 2008). Furthermore, in keeping with the data of Harris et al. (1992), we confirmed that exercise can augment muscle Cr storage (Robinson et al. 1999). Of practical importance, this Cr-mediated augmentation of post-exercise muscle glycogen storage was of a magnitude sufficient to produce a significant improvement in endurance exercise performance (~150 mmol kg−1 dry muscle; Robinson et al. 1999). Although Cr ingestion is well established as a method to improve performance during, and recovery from (in the form of PCr re-synthesis), short-term maximal exercise (Greenhaff et al. 1993, 1994), the work of Robinson et al. (1999) highlighted for the first time the potential beneficial effect of Cr supplementation on the performance of, and maximising recovery from, prolonged sub-maximal exercise. The findings of Robinson et al. (1999) have since been supported by rodent (Op’t Eijnde et al. 2001a) and human (Op’t Eijnde et al. 2001b; Derave et al. 2003; Van Loon et al. 2004) based investigation. The time-course of this Cr-mediated effect on glycogen storage remains unknown, but based on the findings of Robinson et al. (1999) must occur somewhere between 6 and 120 h of post-exercise Cr ingestion. Similarly, insight is lacking into the mechanisms that underpin this phenomenon.
There are a number of potential mechanisms that might underpin the ability of dietary Cr supplementation to augment post-exercise muscle glycogen storage. First, a Cr-induced increase in post-exercise insulin release and/or increase in muscle insulin sensitivity. This hypothesis could be tested by performing an oral-glucose tolerance test (oral-GTT) before exercise and at several pre-determined time points during recovery from exercise, during which volunteers ingest Cr and CHO or CHO alone, and blood glucose and serum insulin are determined. Furthermore, depending upon the time-course of the glycogen super-compensatory response, a Cr-induced augmentation in muscle sarcolemmal glucose transporter (GLUT4) might occur in tandem with these responses (Op’t Eijnde et al. 2001b). Second, an increase in post-exercise glycogen storage could be directly linked to a Cr-induced increase in muscle water content. Indeed, osmotically induced swelling of rat muscle fibres in vitro has demonstrated that muscle glycogen synthesis can be increased by changes in cell volume (Low et al. 1996). Taken in tandem with the decreases in urinary volume and increases in fat-free body mass often reported following Cr ingestion (Hultman et al. 1996; Dentowski et al. 1997), if muscle Cr accumulation (which is sodium dependent) does result in cell swelling in vivo in humans (Francaux and Poortmans 1999), then this may indeed underpin an increase in post-exercise glycogen storage. This could be tested by determining Cr-mediated alterations in muscle water content in tandem with changes in the expression of transcription factors known to be sensitive to osmotic stress responses in humans, for example, c-fos and the inducible 70 kDa heat shock protein (HSP72; Locke 1997; Sadoshima and Izumo 1997; Beck et al. 2000). Finally, given exercise-induced muscle glycogen depletion is known to accompany increased muscle 5′AMP activated protein kinase (AMPK) activation, which in turn has been linked to the mechanistic regulation of post-exercise muscle glucose disposal and storage (Wojtaszewski et al. 2003; Steinberg et al. 2006), it is plausible that a Cr-mediated alteration in exercise-induced AMPK activation could modulate post-exercise muscle glycogen storage. Whilst AMPK is principally activated through changes in the AMP/ATP ratio (Hardie 2004; Hardie and Hawley 2001), a change in the PCr/Cr ratio, which is known to accompany Cr feeding in human muscle (Harris et al. 1992; Robinson et al. 1999), is also known to modulate AMPK activity (Ponticos et al. 1998). Furthermore, AMPK has been reported to be activated indirectly by changes in cell osmotic stress (Hayashi et al. 2000; Fryer et al. 2002).
The primary aim of the present study, therefore, was to extend the findings of Robinson et al. (1999) and to delineate the time-course of Cr-mediated post-exercise muscle glycogen super-compensation in humans, but under more stringent and controlled experimental conditions. We also hoped to provide some insight into potential mechanisms that could underpin this Cr-mediated phenomenon.
Methods
Subjects
Fourteen recreationally active (non-highly trained) and non-vegetarian healthy men (age 26 ± 2 years; height 180 ± 1 cm; body mass 78.6 ± 3.9 kg; body mass index 24.5 ± 1.0 kg m−2; VO2peak 44.4 ± 1.5 ml kg−1 body mass min−1), with no history of prior Cr supplementation, volunteered to participate in the present study. The study was approved by the University of Nottingham Medical School Ethics Committee and conformed to the Declaration of Helsinki. Subjects were advised of the rationale, associated risks and procedures of the study and were aware that they were free to withdraw from the study at any time. Before commencing the investigation, all subjects gave informed written consent, and routine blood and physiological measurements were performed to assess health status prior to acceptance into the study.
Maximal oxygen uptake determination
Upon satisfying the screening criteria, each subject visited the laboratory on two occasions during which they were familiarised with the experimental procedures to be used throughout the study and their peak oxygen uptake (VO2peak) was measured and confirmed. The determination of VO2peak involved the use of an online gas analysis system (SensorMedics, Anaheim, CA, USA) and a continuous incremental exercise protocol on an electrically braked bicycle ergometer at a pedalling cadence of ~70 rpm (Excaliber Sport, Lode N.V. Instrumenten, Groningen, The Netherlands).
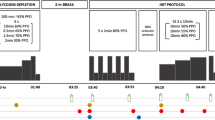
Experimental protocol (Fig. 1)
Subjects reported to the laboratory on the morning of the study after an overnight fast, having abstained from alcohol and strenuous exercise for a minimum of 48 h. Upon arrival, subjects were weighed and then rested in a supine position with their non-dominant hand placed in a hand-warming unit (in which air temperature was maintained at 55 °C) to arterialise the venous drainage of the hand (Gallen and Macdonald 1990). After 20 min, a cannula was inserted into an antecubital vein on the dorsal surface of the subject’s hand, and the hand was returned to the hand-warming unit. The cannula was kept patent using an isotonic saline drip. Subjects then underwent a 2-h oral-glucose tolerance test (GTT), where 90 g of simple CHO was ingested in a 500 ml solution (<2 min), with arterialised-venous blood samples being taken immediately prior to CHO ingestion and 15, 30, 45, 60, 80, 100 and 120 min post-ingestion for the subsequent analysis of the whole blood glucose and lactate (YSI 2300 Statplus analyser, YSI, Yellow Springs, OH, USA), non-esterified free fatty acid (NEFA C kit, Wako Chemicals, Wako, Germany) and serum insulin (Diagnostic Products, Los Angeles, CA, USA) concentrations. Throughout the oral-GTT subjects continued to rest in a supine position and their hand remained in the hand-warming unit to ensure venous arterialisation was maintained.
Upon completion of the oral-GTT, the cannula was removed and subjects immediately began exercising on an electrically braked bicycle ergometer (Excaliber Sport, Lode N.V. Instrumenten, Groningen, The Netherlands) at 70 % VO2peak and a pedal cadence of 70 rpm. Expired gas composition and volume was measured throughout the first 15 min of exercise to confirm subjects were exercising at the correct workload. Perceived exertion was assessed every 15 min during exercise using the Borg scale (Borg 1982). To prevent excessive dehydration during exercise, subjects received 2 ml of water kg−1 body mass every 20 min throughout the exercise period. Subjects exercised continuously until they could no longer maintain a cycling cadence of 70 rpm and wished to stop. At this point, they were allowed to rest supine on a bed for 5 min, and then continued to exercise, interleaved with short rest periods, until the required cycling cadence could no longer be maintained for 2 min or the subject chose not to continue (point of exhaustion). This glycogen-depleting protocol has been used previously by this research laboratory to achieve almost complete muscle glycogen depletion (Casey et al. 1996).
Muscle biopsy sampling and treatment groups (Fig. 1)
At the point of exhaustion, subjects stepped away from the exercise apparatus and rested supine on a bed, whilst a muscle biopsy was immediately obtained from the vastus lateralis of the non-dominant leg using the Bergström needle biopsy technique (Bergström 1975). Upon removal from the limb, the muscle biopsy sample was snap frozen in liquid nitrogen and stored under liquid nitrogen for analysis at a later date. Subjects were then randomly assigned to either a Cr monohydrate (20 g day−1) or placebo (glycine 20 g day−1) treatment group for the remainder of the day and subsequent 5 days. A further muscle biopsy, followed immediately by an oral-GTT, was performed after 1, 3 and 6 days of Cr and placebo ingestion. To avoid potential confounding metabolic effects arising from multiple biopsy sampling over the course of the study, biopsy sites were separated by at least 2.5 cm (Murton et al. 2014).
The Cr treatment group (n = 7) ingested 5 g of Cr (Cr monohydrate; AlzChem, Trostberg, Germany) on four equally spaced occasions each day over a 6 day period, whilst the placebo group (n = 7) received 5 g of glycine (Ajinomoto Inc., Tokyo, Japan) at the same time points. Each 5 g supplement (dissolved in 250 ml of a warm, sugar-free, diluted orange drink) was consumed, and immediately followed by the ingestion of 500 ml of a commercially available CHO-rich drink (Lucozade Original ≈18.5 % w/v glucose and simple sugars, Smith-Kline Beecham, Coleford, UK). This supplementation protocol ensures each Cr supplement is fully dissolved, and the peak in plasma Cr concentration coincides with peak elevations in blood glucose and serum insulin concentration (Green et al. 1996a, b). The CHO dose was chosen because it is known to stimulate muscle Cr and glycogen accumulation in young, healthy volunteers (Green et al. 1996a, b; Robinson et al. 2000). Supplement ingestion commenced immediately after the post-exercise muscle sample (1st biopsy). Before leaving the laboratory, subjects consumed a second treatment (supplement), as detailed previously, and a further two supplements were ingested at intervals over the remainder of that day. Typically, the supplements were ingested at ~12, 2, 6 and 10 pm daily. Subjects repeated the ingestion of the supplements four times each day for a further 5 days. In addition to the supplements, subjects consumed a commercially prepared CHO-rich diet (‘Be Good To Yourself™’ food range, Sainsburys, UK), which when added to the 2 l of Lucozade consumed each day, equated to greater than 80 % of the calorific intake being in the form of CHO throughout the 6 day treatment period (~8 g CHO kg−1 day−1). As subjects received 1350 kcal per day from the Lucozade drinks alone, to ensure that protein intake was not reduced from recommended levels, subjects received 20 % more calories per day than required based on their body mass (37.5 kcal kg−1 body mass day−1). This regimen of Cr ingestion has been widely used and has been proven to have no adverse side effects (Robinson et al. 2000), to increase plasma Cr concentration to a peak of ~700 µmol l−1 when co-ingested with CHO (Green et al. 1996a) and to markedly increase muscle total-Cr content.
Twenty-four h urine collections were made over the first day of supplementation (5 l containers, containing 5 ml of 0.6 mol l−1 thymol solution). Urine volume was recorded and following mixing; an aliquot was removed and stored at −80 °C for future HPLC analysis of urinary Cr and creatinine content (Dunnett et al. 1991).
Muscle sample treatment and analysis
All biopsy samples were divided into two equal portions under liquid nitrogen. Subsequently, one portion was freeze-dried, dissected free from visible blood and connective tissue and powdered. Total muscle water content of samples was determined by weighing the samples before and after freeze-drying. Freeze-dried samples were then extracted in 0.5 M perchloric acid containing 1 mmol EDTA, with the resulting supernatant neutralised with 2.2 M KHCO3 and used for the spectrophotometric determination of glucose-6-phosphate (G-6-P), ATP, PCr and Cr (Harris et al. 1974). Freeze-dried muscle powder was also used for the determination of muscle glycogen (Harris et al. 1974).
Total-RNA was extracted from the remaining portion of frozen (wet) muscle using the method of Chomczynski and Sacchi (1987) and quantified using a kit (Molecular Probes RNA quantification reagent and kit, Cambridge BioScience, Cambridge, UK). Following this, 3 µl of total-RNA was diluted in 0.05 µg µl−1 RNA solution for the subsequent analysis of gene expression.
Following this, 0.5 µg of RNA was added to an Eppendorf tube containing random-hexamers (Promega, Southampton, UK) and RNase-free water, mixed and incubated within a thermal cycler (Mastercycler® Gradient, Eppendorf, Hamburg, Germany) for 5 min at 70 °C and then placed on ice. Reverse transcriptase buffer, dATP, dCTP, dGTP, dTTP and dUTP mix, ribonuclease inhibitor, MMLV reverse transcriptase (Promega, Southampton, UK) and RNase-free water were then added to each sample. Samples were then mixed and incubated at 42 °C for 1 h. Following incubation, α-actin, GLUT4, c-fos and HSP72 mRNA was quantified by real-time PCR using an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Warrington, UK). Probes and primers were designed using Primer Express™ software Version 2.0 (Applied Biosystems, Warrington, UK; Table 1), and all samples were run in triplicate. Results were expressed as ratios towards α-actin, which was considered an endogenous mRNA control allowing variation due to RNA extraction as well as RT (cycle times) efficiencies to be taken into account. Final normalising involved attributing a value of 100 % to the average of the triplicate of determinations for the pre-supplementation (post-exercise) time point.
Calculations and statistics
All data are reported as means ± SEM. Comparisons between treatments, for both absolute concentrations and changes from basal, were carried out using the two-way analysis of variance (ANOVA) with repeated measures. When a significant F value was obtained (P < 0.05), an LSD post hoc test was used to locate any differences (SPSS Base 8.0). Significance was accepted at the 5 % level, unless otherwise stated in the text.
With the exception of lactate, the content of all muscle metabolites was adjusted to the mean ATP concentration within each individual (based upon four biopsy samples). By this means, it was possible to compensate for any admixture of connective tissue and other non-muscular elements within each muscle biopsy sample (Harris et al. 1992).
Glucose, lactate, insulin and non-esterified fatty acid data (NEFA), shown as area under the curve, were calculated from the individual glucose, lactate, serum insulin and NEFA blood concentrations of each subject at ~15 min intervals (0, 15, 30, 45, 60, 80, 100 and 120 min) during the 120 min of the oral-GTT on the treatment days indicated (Kaleidagraph, Synergy Software, Reading, USA).
Results
Subjects reported compliance with all exercise, supplementation and dietary aspects of the study and did not report any ill effects. Maximal oxygen uptake, daily energy intake and dietary composition were identical between treatment groups (Table 2). Urinary Cr excretion was negligible during the first 24 h of placebo supplementation. However, Cr excretion increased dramatically during day 1 of Cr supplementation and was significantly greater than that observed in the placebo group (Cr 0–24 h = 7.6 ± 1.5 g vs. placebo 0–24 h = 0.0 ± 0.4 g; P < 0.01), pointing to ~60 % of the 20 g Cr ingested being retained by the body during the first 24 h of supplementation. No differences in urinary creatinine excretion existed between groups during the first day of Cr or placebo supplementation (Cr 0–24 h = 1.2 ± 0.3 g vs. placebo 0–24 h = 0.9 ± 0.3).
No difference in muscle water content from pre-supplementation (post-exhaustive exercise) existed within or between treatment groups throughout 6 days of placebo or Cr supplementation (Table 3).
Muscle metabolites
No change in muscle ATP or G-6-P content was observed from the pre-supplementation (post-exercise) time point during 6 days of Cr and placebo supplementation (Table 3). Similarly, no differences in muscle ATP and G-6-P content existed between treatment groups over the time-course of the study (Table 3).
No significant change in muscle PCr, free-Cr or total-Cr content over time from the pre-supplementation time point was observed with placebo supplementation (Table 3). There was a 17 % significant increase in muscle PCr content from the pre-supplementation value following 3 days of Cr supplementation (P < 0.05), which increased further following 6 days of Cr ingestion (P < 0.01, Table 3). Creatine ingestion increased muscle PCr content above placebo following 6 days of supplementation (P < 0.01, Table 3). Similarly, Cr ingestion increased muscle free-Cr content following 3 days of supplementation (P < 0.01, Table 3), which was 39 % (P < 0.01) greater than the pre-supplementation value after 6 days of Cr ingestion (Table 3). Creatine ingestion increased muscle free-Cr content above placebo after 3 days of supplementation (P < 0.01), which continued to increase to 35 % greater than placebo following 6 days of Cr ingestion (P < 0.01, Table 3).
In accordance with these changes, muscle total-Cr content was greater than the pre-supplementation value following 1 (9 %, P < 0.05), 3 (14 %, P < 0.01) and 6 (24 %, P < 0.01) days of ingestion (Fig. 2). Creatine ingestion increased muscle total-Cr content above placebo after 1 (8 %, P < 0.05), 3 (11 %, P < 0.01) and 6 (22 %, P < 0.01) days of Cr ingestion (Fig. 2).
Skeletal muscle total-creatine (TCr) content during 6 days of creatine + carbohydrate (Creatine, n = 7) or glycine + carbohydrate (Placebo, n = 7) supplementation following glycogen-depleting exercise in man. Results are expressed as means ± SEM with units of mmol kg−1 dry muscle. Different from the pre-supplementation time point (post-exercise, time 0) within the same treatment group († P < 0.05, †† P < 0.01); different from placebo at the corresponding time point (*P < 0.05, **P < 0.01)
No alteration in the PCr:Cr ratio occurred throughout the study in the placebo group. However, the PCr:Cr ratio was lower than the pre-supplementation value (2.00 ± 0.13) after 3 days Cr supplementation (1.81 ± 0.06, P < 0.05). This value was also lower than that recorded in the placebo group after 3 days supplementation (2.05 ± 0.03, P < 0.05).
The exercise intervention markedly reduced skeletal muscle glycogen content from the habitual resting value of ~400–450 mmol kg−1 dry muscle, and there was no difference in the post-exercise value between treatment groups immediately prior to Cr and placebo ingestion (Fig. 3). Muscle glycogen content increased dramatically in both treatment groups during 6 days of supplementation, and as expected muscle glycogen content was greater than the post-exercise value in both treatment group following 1, 3 and 6 days (Fig. 3). However, Cr supplementation increased muscle glycogen content significantly above placebo after 1 (P < 0.01) and 6 (P < 0.01) days (Fig. 3), with the augmentation of glycogen storage being almost exclusively confined to the initial 24 h of Cr supplementation, and the difference between treatments being maintained thereafter (P < 0.01, Fig. 3). Indeed, the magnitude of glycogen re-synthesis during the first 24 h of supplementation was ~82 % greater in the Cr group compared to placebo (Cr 410 ± 50 vs. placebo 225 ± 50 mmol kg−1 dry muscle, P < 0.01), with no difference in the rate of glycogen synthesis existing between groups between 1 and 6 days of supplementation (Fig. 3).
Skeletal muscle glycogen content during 6 days of creatine + carbohydrate (Creatine, n = 7) or glycine + carbohydrate (Placebo, n = 7) supplementation following glycogen-depleting exercise in man. Results are expressed as means ± SEM with units of mmol kg−1 dry muscle. Different from the pre-supplementation (post-exercise, time 0) time point within the same treatment group († P < 0.05, †† P < 0.01); different from placebo at the corresponding time point (*P < 0.05, **P < 0.01)
Blood metabolites and oral-glucose tolerance tests
The area under the blood glucose-time curve during the oral-GTT (defined in Methods) was unchanged from the pre-supplementation value throughout 6 days of placebo ingestion (Fig. 4a). Creatine supplementation transiently elevated the area under the glucose curve from basal after 1 day of ingestion (P < 0.05, Fig. 4a), but no differences in the area under the curve existed between treatment groups at any time point throughout the study (Fig. 4a).
Area under the curve during an oral glucose tolerance test (GTT) for blood glucose (a), serum insulin (b) and blood lactate (c) before (pre-exercise) and after 1, 3 and 6 days of Cr + carbohydrate or glycine + carbohydrate supplementation following glycogen-depleting exercise in man. Results are expressed as means ± SEM. Area under plasma glucose and lactate curve expressed as (mmol l−1 min−1), area under serum insulin curve expressed as (mU l−1 min−1). † different from the pre-exercise time point within the same treatment group (P < 0.05); * different from placebo group at the corresponding time point (P < 0.05)
Neither placebo nor Cr ingestion had an effect on the area under the serum insulin-time curve during the oral-GTT throughout the study (Fig. 4b). Similarly, no difference between treatment groups was observed at any time point during 6 days of ingestion (Fig. 4b). There was a weak trend for Cr supplementation to increase the serum insulin concentration above placebo after 1 day of supplementation (Placebo day 1 = 4310 ± 642 vs. Cr day 1 = 5995 ± 733 mU/l/min, P = 0.11, Fig. 4b).
The area under the blood lactate-time curve during the oral-GTT was unchanged from pre-supplementation throughout 6 days of placebo ingestion (Fig. 4c). Creatine supplementation increased the area under the blood lactate-time curve from pre-supplementation after 1 (P < 0.05), 3 (P < 0.05) and 6 (P < 0.05) days of ingestion (Fig. 4c). The area under the blood lactate-time curve was also elevated above placebo after 3 (P < 0.05) and 6 (P < 0.05) days of Cr ingestion (Fig. 4c).
Placebo and Cr supplementation both caused a significant reduction in area under blood non-esterified free fatty acid-time curve during the oral-GTT (data not shown). However, no difference existed between treatment groups at any point over the course of the study.
mRNA expression
No differences in GLUT4 mRNA expression existed between placebo and Cr treatment groups throughout 6 days of supplementation (Table 4). However, GLUT4 mRNA expression increased ~twofold from the post-exercise time point following 1 and 6 days of placebo supplementation (P < 0.05; Table 4). No alteration in GLUT4 expression was observed from the post-exercise time point throughout 6 days of Cr supplementation (Table 4).
c-fos mRNA expression was markedly reduced from the post-exercise (pre-supplementation) time point throughout 6 days of supplementation in both treatment groups (P < 0.01; Table 4) However, no differences in expression were evident between treatment groups throughout the study (Table 4).
No differences in HSP72 mRNA expression existed between treatment groups throughout 6 days of supplementation (Table 4). However, HSP72 expression was reduced from the post-exercise time point following 3 days of supplementation in both treatment groups (P < 0.05; Table 4). The reduced expression of HSP72 was transient in the placebo group, but was sustained after 6 days of Cr supplementation (P < 0.05; Table 4).
Discussion
The primary aim of the present study was to delineate the time-course of dietary Cr-mediated post-exercise muscle glycogen super-compensation in humans under conditions, where energy intake and food consumption were stringently controlled. The principal finding was that dietary Cr supplementation markedly augmented post-exercise muscle glycogen storage above placebo during a conventional ‘carbohydrate-loading’ regime, and that this augmentation of glycogen storage occurred almost exclusively within the first 24 h of supplementation (the magnitude of glycogen re-synthesis during the first 24 h of supplementation was ~82 % greater in the Cr group compared to placebo). The results also point to this Cr-induced glycogen super-compensation being independent of the time-course of muscle Cr accumulation and changes in serum insulin availability, insulin sensitivity and/or cellular osmotic stress responses, such that another mechanism(s) must be causative.
Muscle creatine and glycogen storage
Several studies have evaluated muscle glycogen storage in humans in response to acute (20 g day−1 for 5 days; Robinson et al. 1999; Newman et al. 2003; van Loon et al. 2004) and more prolonged Cr ingestion (2.5–20 g day−1 for 8–12 weeks; Op’t Eijnde et al. 2001b; Derave et al. 2003). The findings of these studies largely support a role for dietary Cr in enhancing muscle glycogen storage (+12–23 %) when ingested following exhaustive exercise (Robinson et al. 1999), or during 6–8 weeks of resistance training (+30–35 %; Op’t Eijnde et al. 2001b; Derave et al. 2003). The evidence that dietary Cr ingestion can augment muscle glycogen storage in the non-exercised state is less compelling, with a positive effect (+18 %) being reported by van Loon et al. (2004) following 5 days of Cr supplementation, but no effect at all being reported by others (Newman et al. 2003; Sewell et al. 2008), even within the same volunteer when one limb has been exercised and the contra-lateral limb has remained inactive (Robinson et al. 1999). As far as we are aware, the present study is the first to establish the post-exercise glycogen super-compensatory properties of dietary Cr supplementation under rigorously controlled dietary conditions in humans (Table 2) and to document the time-course of this effect. It is clear from the data (Fig. 3) that the Cr-induced augmentation of muscle glycogen storage occurs not as a gradual bifurcation from the typical glycogen-loading response observed in the placebo group, but rather as a transient marked increase (approximately twofold) in glycogen re-synthesis during the initial 24 h of Cr ingestion during recovery from exercise (Fig. 3).
It has been suggested that the expansion of the muscle total-Cr pool is a prerequisite for Cr-induced augmentation of muscle glycogen storage (Van Loon et al. 2004; Volek and Rawson 2004). Indeed, work by Van Loon et al. (2004) showed a significant correlation between the magnitude of increase in muscle total-Cr (+31 %) and glycogen storage (+18 %) following 5 days of Cr supplementation. This observation was strengthened by the findings from another study of this research group, where the absence of a treatment effect upon glycogen storage was attributed to inadequate muscle Cr accumulation (+12 %; Newman et al. 2003). However, in neither of these studies were muscle biopsies obtained over the time-course of Cr supplementation. Indeed, as the Cr-mediated augmentation of glycogen storage in the present study occurred almost exclusively within the first 24 h of Cr ingestion, when muscle total-Cr stores had increased by only 8 % (Fig. 2), the findings clearly do not support the contention that the extent of muscle total-Cr accumulation is an important determinant of the muscle glycogen storage response. Furthermore, following 24 h, no greater muscle glycogen storage above that recorded in the placebo group was observed from days 1 to 6 of Cr ingestion, despite muscle total-Cr increasing by a further 15 % in the Cr supplementation group (Figs. 2, 3).
It could be speculated that a transient change in the PCr/Cr ratio in response to 24 h of Cr feeding may have blunted the exercise-induced activation of AMPK (Winder and Hardie 1999; Rasmussen and Winder 1997; Fujii et al. 2000), which in turn, and independent of any alteration in muscle insulin sensitivity, could have augmented muscle glycogen synthesis during the initial 24 h of post-exercise recovery by dampening the inhibitory effect of AMPK on glycogen synthase activity (Aschenbach et al. 2002; Wojtaszewski et al. 2002; Jorgensen et al. 2004). Indeed, an alteration in the composition of the muscle Cr pool, as occurs, for example, during exercise and Cr feeding, has been reported to directly impact upon the activation status of AMPK (Ponticos et al. 1998). The in vitro findings of Ponticos et al. (1998) showed that physiological increases in muscle PCr content directly inhibited AMPK activation in a dose-dependent manner. Furthermore, this inhibition was attenuated (indirectly) by an increase in muscle free-Cr content; pointing to the PCr/Cr ratio, along with the ATP/ADP ratio, being a regulator of skeletal muscle AMPK activation status (for review, see Hardie and Hawley 2001). A reduction in the muscle PCr/Cr ratio has been reported following acute-Cr ingestion (Harris et al. 1992; Robinson et al. 1999), which has prompted researchers to examine the effect of Cr availability on AMPK activation in vitro (Ceddoa and Sweeny 2004) and in vivo (Ju et al. 2005; Op’t Eijnde et al. 2005). The findings from these studies are equivocal, with AMPK being activated by unphysiological concentrations of PCr and Cr (Ceddoa and Sweeny 2004), and independent of any alteration in the muscle PCr/Cr ratio (Ju et al. 2005), in some, but not all, studies (Op’t Eijnde et al. 2005). In the present in vivo study, Cr ingestion reduced the muscle PCr/Cr ratio from basal following 3 days of supplementation, but this response occurred after the Cr-mediated increase in muscle glycogen storage (0–24 h, Fig. 3), making it unlikely that this underpinned the augmentation of glycogen storage observed between 0 and 24 h. Furthermore, contrary to the report of Ponticos et al. (1998), more recent in vitro research has surmised that although the concept of a PCr/Cr ratio mediated regulation of AMP kinase may be intuitive and attractive, evidence to substantiate this concept is not prevalent (Taylor et al. 2006; Suter et al. 2006).
Muscle creatine and insulin sensitivity
It is logical to assume that the rapid augmentation of post-exercise glycogen storage that accompanied Cr ingestion (Fig. 3) would have been preceded by an increase in muscle glucose uptake, which we hoped to demonstrate by performing oral-GTTs and determining muscle GLUT4 mRNA expression (Op’t Eijnde et al. 2001b) over the course of 6 days of exercise recovery. However, given that the Cr-induced augmentation of muscle glycogen storage occurred almost exclusively within the first 24 h of treatment and, therefore, preceded the first post-exercise oral-GTT (1 day), any effect of Cr ingestion on glucose clearance must have occurred within the initial 24 h of recovery. Indeed, based upon our previous observation (Robinson et al. 1999), which found no effect of Cr ingestion on muscle glycogen storage during the initial 6 h of recovery from exercise, and the present study that showed a dramatic effect after 24 h of recovery (Fig. 3), this Cr augmentation of glycogen storage must have occurred between 6 to 24 h of post-exercise recovery. The increase in area under the blood glucose curve during the oral-GTT after 1 day of Cr ingestion (Fig. 4a), and along with the trend for the serum insulin response to do the same (Fig. 4b), most probably reflects a blunting in muscle glucose uptake caused by the marked increase in muscle glycogen storage over the preceding 24 h. This view is supported by the increase in blood lactate concentration that accompanied Cr ingestion (Fig. 4c).
It has been suggested that Cr supplementation can up-regulate muscle GLUT4 protein expression, thereby increasing muscle glucose uptake and underpinning the glycogen super-compensatory properties of Cr supplementation (Op’t Eijnde et al. 2001b). However, this view is not supported by the findings others (Op’t Eijnde et al. 2001a; van Loon et al. 2004), where, for example, no increase in muscle GLUT4 mRNA or protein expression was observed in response to acute or prolonged Cr ingestion in healthy human volunteers (van Loon et al. 2004). The finding of the present study that Cr ingestion had no influence on muscle GLUT4 mRNA expression (Table 4) is in concordance with this stance. However, it is acknowledged that the quantification of muscle GLUT4 protein and/or components of the signalling cascade regulating GLUT4 translocation (e.g., AS160 activation) would have provided more robust insight.
Muscle cell volume and osmotic stress responses
Another mechanism that has been suggested to underpin the stimulatory effect of Cr ingestion on acute glycogen super-compensation is an increase in intra-myocellular water content, secondary to an increase in muscle Cr accumulation (Robinson et al. 1999; Op’t Eijnde et al. 2001b; van Loon et al. 2004). Indeed, muscle Cr transport is dependent upon extracellular Na+, and is, therefore, osmotically active (Daly and Seifter 1980), with an increase in fat-free body mass and a decrease in urinary volume being reported in response to acute-Cr feeding; indicative of an increase whole-body water retention (Hultman et al. 1996). It has been demonstrated in vitro that changes in cell volume in rodent muscle can modulate changes in muscle glycogen content, independent of any alteration in cell glucose uptake (Low et al. 1996). However, although muscle total-Cr (Fig. 2) and glycogen (Fig. 3) both increased during the initial 24 h of dietary Cr ingestion in the present study, the time-course of change for each metabolite over the 6 days of supplementation was very different, with the glycogen super-compensatory response being almost exclusively restricted to the initial 24 h of supplementation, whilst muscle total-Cr content increased in a more gradual manner over the study. It is unlikely, therefore, that a Cr-mediated increase in cellular hydration status stimulated this marked initial increase in muscle glycogen storage, such that another mechanism seems likely. This conclusion is supported by the lack of any treatment effect on muscle water content during 6 days of supplementation (Table 3) and by the absence of any differences in mRNA expression of the osmotically induced transcription factors c-fos and HSP72 between Cr and placebo groups (Table 4). Changes in the expression of HSP72 (Febraio et al. 2002) and c-fos (Puntschart et al. 1998) mRNA have been shown to correlate well with changes in their respective protein products, indicating they are at least partly under transcriptional regulation. Furthermore, it has been suggested that muscle HSP72 expression is sensitive to alterations in myofibril glycogen content in man, with HSP72 mRNA increasing in response to glycogen-depleting exercise before gradually being reduced as glycogen stores are replenished (Febbraio and Koukoulas 2000; Febraio et al. 2002). Although the present study lacked a pre-exercise muscle biopsy, in support of the findings of Febraio et al. (2002), post-exercise HSP72 expression was halved following 3 days of recovery in the Cr and placebo groups, as muscle glycogen was super-compensated (Table 4). However, it is clear that HSP72 mRNA expression was insensitive to the marked differences in muscle glycogen content that existed between treatment groups following 1 day of supplementation (Fig. 3; Table 4).
In conclusion, we have confirmed the ability of dietary Cr supplementation to augment post-exercise muscle glycogen storage during a conventional and rigorously controlled ‘carbohydrate-loading’ regimen in humans. Furthermore, this Cr-induced augmentation of muscle glycogen storage occurred almost exclusively within the first 24 h of exercise recovery and Cr ingestion, and appeared to be unrelated to changes in the magnitude of increase in muscle total-Cr, PCr or Cr content, muscle insulin sensitivity, or osmotic stress and muscle cell volume responses, such that another mechanism must be causative.
References
Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ (2002) Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 51:567–573
Beck F-X, Grünbein R, Lugmayr K, Neuhofer W (2000) Heat shock proteins and the cellular response to osmotic stress. Cell Physiol Biochem 10:303–306
Bergström J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Bergström J, Hultman E (1966) Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210:309–310
Bergström J, Hultman E (1967) A study of glycogen metabolism during exercise in man. Scand J Clin Lab Invest 19:218–228
Bergström J, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Casey A, Short AH, Curtis S, Greenhaff PL (1996) The effect of glycogen availability on power output and the metabolic response to repeated bouts of maximal, isokinetic exercise in man. Eur J Appl Physiol 72:249–255
Ceddoa RB, Sweeny G (2004) Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol 555:409–421
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Daly MM, Seifter S (1980) Uptake of creatine by cultured cells. Arch Biochem Biophys 203:317–324
Dentowski A, Opaszowski BH, Blanchnio D, Ponanowski B (1997) Effect of creatine supplementation on the performance in supra-maximal, intermittent exercise. Biol Sport 14:291–298
Derave W, Eijnde BO, Verbessem P, Ramaekers M, Van Leemputte M, Richter EA, Hespel P (2003) Combined creatine and protein supplementation in conjunction with resistance training promotes muscle GLUT-4 content and glucose tolerance in humans. J Appl Physiol 94:1910–1916
Dunnett M, Harris RC, Orme CE (1991) Reverse-phase-ion-pairing high-performance liquid chromatography of phosphocreatine, creatine and creatinine in equine muscle. Scand J Clin Lab Investig 51:137–141
Febbraio MA, Koukoulas I (2000) HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol 89:1055–1060
Febraio MA, Steensberg A, Walsh R, Koukoulas I, Van Hall G, Saltin B, Pedersen BK (2002) Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol 583:911–917
Francaux M, Poortmans JR (1999) Effects of training and creatine supplement on muscle strength and body mass. Eur J Appl Physiol 80:165–168
Fryer LGD, Foufelle F, Baines K, Baldwin SA, Woods A, Carling D (2002) Characterisation of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J 363:167–174
Fujii N, Hayashi T, Hirshman MF, Smith TJ, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnhaum MJ, Witters LA, Thorell A, Goodyear LJ (2000) Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun 273:1150–1155
Gallen IW, Macdonald IA (1990) Effect of two methods of hand heating on body temperature, forearm blood flow, and deep venous oxygen saturation. Am J Physiol 259:E639–E643
Green AL, Simpson EJ, Littlewood JJ, Macdonald IA, Greenhaff PL (1996a) Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiologica Scandinavia 158:195–202
Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL (1996b) Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol 271:E821–E826
Greenhaff PL, Casey A, Short AH, Harris R, Söderlund K, Hultman E (1993) The influence of oral creatine supplementation on muscle torque during repeated bouts of maximal voluntary exercise in man. Clin Sci 84:565–571
Greenhaff PL, Bodin K, Söderlund K, Hultman E (1994) The effect of oral creatine supplementation on skeletal muscle phosphocreatine re-synthesis. Am J Physiol 266:E725–E730
Hardie DG, Hawley SA (2001) AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays 23:1112–1119
Hardie DG (2004) AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc 36:28–34
Harris RC, Hultman E, Nordesjö LO (1974) Glycogen, glycolytic intermediates and high energy phosphates determined in biopsy samples of musculus femoris of man at rest. Methods in variance values. Scand J Clin Lab Invest 33:109–120
Harris RC, Södurlund K, Hultman E (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci 83:367–374
Hayashi T, Hirshman MF, Fujii N, Haninowski SA, Witters LA, Goodyear LJ (2000) Metabolic stress and altered glucose transport activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49:527–531
Hultman E, Söderlund K, Timmons JA, Cederlad G, Greenhaff PL (1996) Muscle creatine loading in man. J Appl Physiol 81:232–237
Jentjens R, Jeukendrup AE (2003) Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med 33:117–144
Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JFP (2004) The α2–5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 53:3074–3081
Ju J-S, Smith JL, Oppelt PJ, Fisher JS (2005) Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am J Physiol 288:E347–E352
Locke M (1997) The cellular stress response to exercise: role of stress proteins. Exerc Sports Sci Rev 25:105–136
Low SY, Rennie MJ, Taylor PM (1996) Modulation of glycogen synthesis in rat skeletal muscle by changes in cell volume. J Physiol 495:299–303
Murton AJ, Billeter R, Stephens FB, Des Etages SG, Graber F, Hill RJ, Marimuthu K, Greenhaff PL (2014) Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training. J Appl Physiol 116:113–125
Newman JE, Hargreaves M, Garnham A, Snow RJ (2003) Effect of creatine ingestion on glucose tolerance and insulin sensitivity in men. Med Sci Sports Exerc 35:69–74
Op’t Eijnde B, Richter EA, Henquin J-C, Kiens B, Hespel P (2001a) Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiologica Scandinavia 171:169–176
Op’t Eijnde B, Urso B, Richter EA, Greenhaff PL, Hespel P (2001b) Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes 50:18–23
Op’t Eijnde B, Derave W, Wojtaszewski JFP, Richter EA, Hespel P (2005) AMP-kinase expression and activity in human skeletal muscle: effects of immobilisation, retraining and creatine supplementation. J Appl Physiol 98:1228–1233
Ponticos M, Long LuQ, Morgan JE, Hardie DG, Partridge TA, Carling D (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 17:1688–1699
Puntschart A, Wey E, Jostarndt K, Vogt M, Wittwer M, Widmer HR, Hoppeler H, Billeter R (1998) Expression of fos and jun genes in human skeletal muscle after exercise. Am J Physiol 274:C129–C137
Rasmussen BB, Winder WW (1997) Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol 83:1104–1109
Robinson TM, Sewell DA, Hultman E, Greenhaff PL (1999) Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J Appl Physiol 87:598–604
Robinson TM, Sewell DA, Casey A, Steenage G, Greenhaff PL (2000) Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med 34:284–288
Sadoshima J, Izumo S (1997) Tyrosine kinases mediation of c-fos expression by cell swelling in cardiac myocytes. Heart Vessels 12:194–197
Sewell DA, Robinson TM, Greenhaff PL (2008) Creatine supplementation does not affect human skeletal muscle glycogen content in the absence of prior exercise. J Appl Physiol 104:508–512
Sherman WM, Costill DL (1984) The marathon: dietary manipulation to optimize performance. Am J Sports Med 12:44–51
Steinberg GR, Watt MJ, McGee SL, Chan S, Hargreaves M, Febbraio MA, Stapleton D, Kemp BE (2006) Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl Physiol Nutr Metab 31:302–312
Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D (2006) Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281:32207–32216
Taylor EB, Ellingson WJ, Lamb JD, Chesser DG, Compton CL, Winder WW (2006) Evidence against regulation of AMP-activated protein kinase and LKB1/STRAD/MO25 activity by creatine phosphate. Am J Physiol 290:E661–E669
Van Loon LJC, Murphy R, Oosterlaar AM, Cameron-Smith D, Hargreaves M, Wagonmakers AJM, Snow R (2004) Creatine supplementation increases glycogen storage but not GLUT-4 expression in human skeletal muscle. Clin Sci 106:99–106
Volek JS, Rawson ES (2004) Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition 20:609–614
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10
Wojtaszewski JFP, Jørgensen SB, Hellsten Y, Hardie DG, Richter EA (2002) Glycogen dependent effects of AICAR on 5’AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51:284–292
Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA (2003) Regulation of 5’AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol 284:E813–E822
Acknowledgments
The authors would like to thank Dr Michelle Holdsworth for her help in planning the subject diets and Ms Liz Simpson, Dr Nandini Seevaratman and Dr Kishor Patel for providing additional medical support for the study when required. We also wish to thank a dedicated group of subjects for their participation in this study and Glaxo-Smithkline, Coleford, UK, for providing the Lucozade drinks.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was funded as part of the Chemical Biological Defence and Human Sciences Domain of the UK MoD Corporate Research Programme.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: T. Wallimann and R. Harris.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roberts, P.A., Fox, J., Peirce, N. et al. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids 48, 1831–1842 (2016). https://doi.org/10.1007/s00726-016-2252-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2252-x