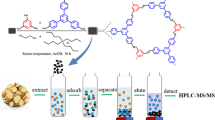
Abstract
Natural products derived from medicinal plants have gained an important role in drug discovery due to their complex and abundant composition of secondary metabolites, with their structurally unique molecular components bearing a significant number of stereo-centers exhibiting high specificity linked to biological activity. Usually, the extraction process of natural products involves various techniques targeting separation of a specific class of compounds from a highly complex matrix. Aiding the process entails the use of well-defined and selective molecular extractants with distinctly configured structural attributes. Calixarenes conceivably belong to that class of molecules. They have been studied intensely over the years in an effort to develop new and highly selective receptors for biomolecules. These macrocycles, which display remarkable structural architectures and properties, could help usher a new approach in the efficient separation of specific classes of compounds from complex matrices in natural products. A simple and rapid such extraction method is presented herein, based on host–guest interaction(s) between a calixarene synthetic receptor, 4-tert-butyl-calix[6]arene, and natural biomolecular targets (amino acids and peptides) from Helleborus purpurascens and Viscum album. Advanced physicochemical methods (including GC–MS and chip-based nanoESI-MS analysis) suggest that the molecular structure and specifically the calixarene cavity size are closely linked to the nature of compounds separated. Incorporation of biomolecules and modification of the macrocyclic architecture during separation were probed and confirmed by scanning electronic microscopy and atomic force microscopy. The collective results project calixarene as a promising molecular extractant candidate, facilitating the selective separation of amino acids and peptides from natural products.








Similar content being viewed by others
References
Atwood JL, Steed JW (2004) Encyclopedia of Supramolecular Chemistry vol.1, CRC Press
Bart HJ, Pilz S (2011) Industrial scale natural products extraction, 1st edn. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Fiehn O, Kopka J, Trethewey RN, Willmitzer L (2000) Identification of uncommon plant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal Chem 72:3573–3580
Gutsche CD (2008) Calixarenes: an introduction, monographs in supramolecular chemistry. Royal Society of Chemistry, Cambridge
Hassen WM, Martelet C, Davis F, Higson SPJ, Abdelghani A, Helali S, Jaffrezic-Renault N (2007) Calix[4]arene based molecules for amino-acid detection. Sens Actuators B 124:38–45
Ikeda A, Shinkai S (1997) Novel cavity design using calix[n]arene skeletons: towards molecular recognition and metal binding. Chem Rev 97:1713–1734
Karpagasundari C, Kulothungan S (2014) Analysis of bioactive compounds in Physalis minima leaves using GC MS, HPLC, UV-VIS and FTIR techniques. J Pharmacogn Phytochem 3:196–201
Koh K, Araki K, Shinkai S, Asfari Z, Vicens J (1995) Cation binding properties of a novel 1,3-alternate calix[4]bis crown formation of 1:1 and 1:2 complexes and unique cation tunneling across a calix[4]arene cavity. Tetrahedron Lett 36:6095–6098
Latterini L, Tarpani L (2012) AFM measurements to investigate particulates and their interactions with biological macromolecules. In: Frewin C (ed) Atomic force microscopy investigations into biology—from cell to protein. InTech, Rijeka, pp 87–98
Leonards PEG, Brix R, Barceló D, Lamoree M (2011) Advanced GC–MS and LC–MS tools for structure elucidation in effect-directed analysis. In: Brack W (ed) Effect-directed analysis of complex environmental contamination, the handbook of environmental chemistry, vol 15. Springer, Berlin, pp 143–165
Ludwig R (2000) Calixarenes in analytical and separation chemistry. Fresenius J Anal Chem 367:103–128
Ludwig R (2005) Calixarenes for biochemical recognition and separation. Microchim Acta 152:1–19
Ludwig R, Thi Kim Dzung N (2002) Calixarene-based molecules for cation recognition. Sensors 2:397–416
Mutihac L, Buschmann HJ, Mutihac RC, Schollmeyer E (2005) Complexation and separation of amines, amino acids and peptides by functionalized calix[n]arenes. J Incl Phenom Macrocyclic Chem 51:1–10
Neda I, Vlazan P, Pop RO, Sfirloaga P, Grozescu I, Segneanu AE (2012) Peptide and amino acids separation and identification from natural products. In: Krull IS (ed) Analytical chemistry. InTech, Rijeka, pp 135–146
Oshima T, Goto M, Furusaki S (2002) Extraction behavior of amino acids by calix[6]arene carboxylic acid derivates. J Incl Phenom Macrocyclic Chem 43:77–86
Popescu C, Fitigǎu F, Segneanu AE, Martagiu R, Vaszilcsin CG (2011) Separation and characterization of anthocyanins by analytical and electrochemical methods. Environ Eng Manag J 10(5):697–701
Shimojo K, Oshima T, Goto M (2004) Calix[6]arene acetic acid extraction behavior and specificity with respect to nucleobases. Anal Chim Acta 521:163–171
Sikorka M, Matlawska I, Glowniak K, Zgorka G (2000) Qualitative and quantitative analysis of phenolic acids in Asclepias Syriaca L. Acta Pol Pharm Drug Res 57:69–72
Sirit A, Yilmaz M (2009) Chiral calixarenes. Turk J Chem 33:159–200
Stone MM, Franz AH, Lebrilla CB (2002) Non-covalent calixarene amino acid complexes formed by MALDI-MS. J Am Soc Mass Spectr 13:964–974
Vaszilcsin CG, Segneanu AE, Balcu I, Fitigǎu F, Mirica MC (2010) Eco-friendly extraction and separation methods of capsaicines. Environ Eng Manag J 9(7):971–976
Villas-Bôas SG, Smart K, Sivakumaran S, Lane GA (2011) Alkylation or silylation for analysis of amino and non-amino organic acids by GC-MS? Metabolites 1:3–20
Xu JZ, Shen J, Cheng Y, Qu H (2009) GC-MS analysis of amino acids in extract of Cornus Caprae hircus. Chem Res Chin Univ 25:812–816
Yilmaz M, Erdemir S (2013) Calixarene-based receptors for molecular recognition. Turk J Chem 37:558–585
Acknowledgments
The study was supported by Romania–China Bilateral Grant nr. 628/2013: BIOSIM—“Study on Activity and Potential Drug Interactions of Immunomodulating Natural Products”. We would like to thank Dr. A. D. Zamfir, I. Neda and Dr. M. Birdeanu (INCEMC Timisoara) for their contribution to this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: T. Langer.
Rights and permissions
About this article
Cite this article
Segneanu, AE., Damian, D., Hulka, I. et al. A simple and rapid method for calixarene-based selective extraction of bioactive molecules from natural products. Amino Acids 48, 849–858 (2016). https://doi.org/10.1007/s00726-015-2132-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2132-9