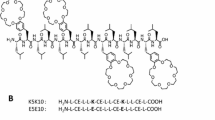
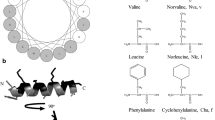
Abstract
Cationic antimicrobial peptides (AMPs) are essential components of the innate immune system, offering protection against invading pathogenic bacteria. In nature, AMPs serve as antibiotics with broad-spectrum antimicrobial and anti-biofilm properties. However, low effective stability in high-salt environments and physiological instability in biological membranes limit the applicability of naturally occurring AMPs as novel therapeutics. We therefore designed short synthetic cationic peptides by substituting key residues in myxinidin, an AMP derived from the epidermal mucus of hagfish, with lysine (Lys, K), arginine (Arg, R), and tryptophan (Trp, W). The resultant myxinidin analogs exhibited strong antimicrobial activity against both Gram-positive and Gram-negative bacteria, including multidrug-resistant strains, even under high-salt conditions. Moreover, these peptides showed high binding affinity for both lipopolysaccharides and lipoteichoic acids and inhibited biofilm formation by most bacteria, but did not cause significant lysis of human red blood cells and were not cytotoxic to normal human keratinocytes. Circular dichroism analysis revealed that myxinidin and its analogs assumed α-helical or β-sheet structures within artificial liposomes and bacterial membranes. In addition, bacterial killing and membrane permeation experiments demonstrated that the myxinidin analogs permeated through bacterial membranes, leading to cytoplasmic disruption and cell death. Taken together, these findings suggest myxinidin analogs may be promising candidate antibiotic agents for therapeutic application against antibiotic-resistant bacteria.












Similar content being viewed by others
References
Bhattachariya S, Ramamoorthy A (2009) Multifunctional host defense peptides: functional and mechanistic insights from NMR structures of potent antimicrobial peptides. FEBS J 276(22):6465–6473
Bi X, Wang C, Ma L, Sun Y, Shang D (2013) Investigation of the role of tryptophan residues in cationic antimicrobial peptides to determine the mechanism of antimicrobial action. J Appl Microbiol 115(3):663–672
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Bruck E (1980) National Committee for Clinical Laboratory Standards. Pediatrics 65(1):187–188
Cantisani M, Leone M, Mignogna E, Kampanaraki K, Falanga A, Morelli G, Galdiero M, Galdiero S (2013) Structure–activity relations of myxinidin, an antibacterial peptide derived from the epidermal mucus of hagfish. Antimicrob Agents Chemother 57(11):5665–5673
Cantisani M, Finamore E, Mignogna E, Falanga A, Nicoletti GF, Pedone C, Morelli G, Leone M, Galdiero M, Galdiero S (2014) Structural insights into and activity analysis of the antimicrobial peptide myxinidin. Antimicrob Agents Chemother 58(9):5280–5290
Chan C, Burrows LL, Deber CM (2004) Helix induction in antimicrobial peptides by alginate in biofilms. J Biol Chem 279(37):38749–38754
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta 1758(9):1184–1202
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22(6):996–1006
Gopal R, Park JS, Seo CH, Park Y (2012) Applications of circular dichroism for structural analysis of gelatin and antimicrobial peptides. Int J Mol Sci 13(3):3229–3244
Gopal R, Lee JK, Lee JH, Kim YG, Oh GC, Seo CH, Park Y (2013a) Effect of repetitive lysine–tryptophan motifs on the eukaryotic membrane. Int J Mol Sci 14(1):2190–2202
Gopal R, Seo CH, Song PI, Park Y (2013b) Effect of repetitive lysine–tryptophan motifs on the bactericidal activity of antimicrobial peptides. Amino Acids 44(2):645–660
Gopal R, Kim YG, Lee JH, Lee SK, Chae JD, Son BK, Seo CH, Park Y (2014) Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother 58(3):1622–1629
Hancock RE, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8(9):402–410
Hou M, Zhang N, Yang J, Meng X, Yang R, Li J, Sun T (2013) Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 32(3):614–623
Jamasbi E, Batinovic S, Sharples RA, Sani MA, Robins-Browne RM, Wade JD, Separovic F, Hossain MA (2014) Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 46(12):2759–2766
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19(3):491–511
Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45(7):1169–1196
Liu YF, Xia X, Xu L, Wang YZ (2013) Design of hybrid b-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 34:237–250
Loh B, Grant C, Hancock RE (1984) Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26(4):546–551
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367(9524):1747–1757
Majer A, Kidric J, Jerala R (2003) Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J Antimicrob Chemother 51:1159–1165
Mayer LD, Hope MJ, Cullis PR (1986) Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858(1):161–168
Mohamed MF, Hammac GK, Guptill L, Seleem MN (2014) Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant Staphylococcus pseudintermedius from infected dogs. PLoS ONE 9(12):e116259
Moore RA, Bates NC, Hancock RE (1986) Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother 29(3):496–500
Nan YH, Lee SH, Kim HJ, Shin SY (2010) Mammalian cell toxicity and candidacidal mechanism of Arg- or Lys-containing Trp-rich model antimicrobial peptides and their d-enantiomeric peptides. Peptides 31(10):1826–1831
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341:501–513
Park Y, Lee DG, Jang SH, Woo ER, Jeong HG, Choi CH, Hahm KS (2003) A Leu–Lys-rich antimicrobial peptide: activity and mechanism. Biochim Biophys Acta 1645(2):172–182
Park IY, Cho JH, Kim KS, Kim YB, Kim MS, Kim SC (2004) Helix stability confers salt resistance upon helical antimicrobial peptides. J Biol Chem 279(14):13896–13901
Park Y, Park SC, Park HK, Shin SY, Kim Y (2007) Hahm KS (2007) Structure–activity relationship of HP(2-20) analog peptide: enhanced antimicrobial activity by N-terminal random coil region deletion. Biopolymers 88(2):199–207
Park SC, Park Y, Hahm KS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci 12(9):5971–5992
Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ (2012) Antimicrobial peptides for therapeutic applications: a review. Molecules 17(10):12276–12286
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2(5):a000414
Strempel N, Strehmel J, Overhage J (2015) Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr Pharm Des 21(1):67–84
Strom MB, Haug BE, Rekdal O, Skar ML, Stensen W, Svendsen JS (2002) Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochemistry and cell biology = Biochimie et biologie cellulaire 80(1):65–74
Subramanian S, Ross NW, MacKinnon SL (2009) Myxinidin, a novel antimicrobial peptide from the epidermal mucus of hagfish, Myxine glutinosa L. Mar Biotechnol 11(6):748–757
Sun J, Xia Y, Li D, Du Q, Liang D (2014) Relationship between peptide structure and antimicrobial activity as studied by de novo designed peptides. Biochim Biophys Acta 1838(12):2985–2993
Takahashi D, Shukla SK, Prakash O, Zhang G (2010) Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 92(9):1236–1241
Tran AX, Whittimore JD, Wyrick PB, McGrath SC, Cotter RJ, Trent MS (2006) The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J Bacteriol 188(12):4531–4541
Trauble H, Overath P (1973) The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta 307:491–512
Wang K, Dang W, Yan J, Chen R, Liu X, Yan W, Zhang B, Xie J, Zhang J, Wang R (2013) Membrane perturbation action mode and structure–activity relationships of Protonectin, a novel antimicrobial peptide from the venom of the neotropical social wasp Agelaia pallipes pallipes. Antimicrob Agents Chemother 57(10):4632–4639
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5(10):905–917
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Acknowledgments
This work was supported by a Global Research Laboratory (GRL) Grant (NRF-2014K1A1A2064460), Chosun University (2014) and Human Resource Training Project for Regional Innovation (NRF-2013H1B8A2032054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: F. Albericio.
H. M. Han and R. Gopal contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, H.M., Gopal, R. & Park, Y. Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties. Amino Acids 48, 505–522 (2016). https://doi.org/10.1007/s00726-015-2104-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2104-0