Abstract
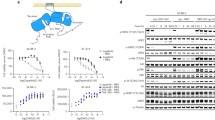
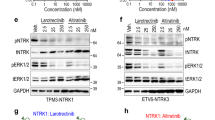
The gatekeeper T798M mutation in HER2 kinase domain has been observed to considerably shift drug sensitivity to HER2 in breast cancer therapy. Here, drug response of clinical tyrosine kinase inhibitors (TKIs) to the mutation was profiled using a synthetic biology protocol. It was found that TKIs can be grouped into three classes in terms of their response behavior to T798M mutation: class I inhibitors exhibit drug resistance upon the mutation, such as lapatinib, TAK-285 and AEE788; class II inhibitors are insensitive to the mutation, such as erlotinib and gefitinib; and class III inhibitors can be sensitized by the mutation, such as staurosporine. However, kinetic study indicated that the mutation has only a modest effect on the binding of substrate ATP to HER2. Binding free energy analysis revealed that the drug response is primarily determined by direct interaction between the kinase and inhibitors, but not by indirect kinase interaction with competitive ATP. This is different to the molecular mechanism of “generic” drug resistance conferring from EGFR gatekeeper T790M mutation, which is caused by increased ATP affinity upon the mutation. Structural analysis of kinase–inhibitor complexes unraveled that HER2 T798M mutation induces significant steric hindrance to class I inhibitors, but can establish additional nonbonded interactions for class III inhibitors.







Similar content being viewed by others
References
Ai X, Sun Y, Wang H, Lu S (2014) A systematic profile of clinical inhibitors responsive to EGFRsomatic amino acid mutations in lung cancer: implication for the molecular mechanism of drug resistance and sensitivity. Amino Acids 46:1635–1648
Barker SC, Kassel DB, Weigl D, Huang X, Luther MA, Knight WB (1995) Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry 34:14843–14851
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Bray F, McCarron P, Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 6:229–239
Caffarel MM, Andradas C, Pérez-Gómez E, Guzmán M, Sánchez C (2012) Cannabinoids: a new hope for breast cancer therapy? Cancer Treat Rev 38:911–918
Chan PM, Nestler HP, Miller WT (2000) Investigating the substrate specificity of the HER2/Neu tyrosine kinase using peptide libraries. Cancer Lett 160:159–169
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Darden T, York D, Pedersen L (1993) Particale mesh Ewald and N.log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
De Grève J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, Everaert H, Umelo I, In’t Veld P, Schallier D (2012) Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 76:123–127
Doi T, Takiuchi H, Ohtsu A, Fuse N, Goto M, Yoshida M, Dote N, Kuze Y, Jinno F, Fujimoto M, Takubo T, Nakayama N, Tsutsumi R (2012) Phase I first-in-human study of TAK-285, a novel investigational dual HER2/EGFR inhibitor, in cancer patients. Br J Cancer 106:666–672
Duan Y, Wu C, Chowdhury S, Lee MC, Xiong G, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang J, Kollman P (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24:1999–2012
Higa GM, Abraham J (2007) Lapatinib in the treatment of breast cancer. Exp Rev Anticancer Ther 7:1183–1192
Homeyer N, Gohlke H (2012) Free energy calculations by the molecular mechanics Poisson–Boltzmann surface area method. Mol Inf 31:114–122
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J (2011) Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS One 6:e26760
Krivov GG, Shapovalov MV, Dunbrack RL Jr (2009) Improved prediction of protein side-chain conformations with SCWRL4. Proteins 77:778–795
Ma C, Wei S, Song Y (2011) T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis 3:10–18
Mitri Z, Constantine T, O’Regan R (2012) The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract 2012:743193
Olayioye MA (2001) Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res 3:385–389
Rexer BN, Ghosh R, Narasanna A, Estrada MV, Chakrabarty A, Song Y, Engelman JA, Arteaga CL (2013) Human breast cancer cells harboring a gatekeeper T798M mutation in HER2 overexpress EGFR ligands and are sensitive to dual inhibition of EGFR and HER2. Clin Cancer Res 19:5390–5401
Rüegg UT, Burgess GM (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharm Sci 10:218–220
Ryckaert J, Ciccotti G, Berendsen HJC (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341
Shen X, Chen B, Ma Z, Xie B, Cao X, Yang T, Zhao Y, Qin J, Li J, Cao F, Chen X (2015) A systematic analysis of the resistance and sensitivity of HER2YVMA receptor tyrosine kinase mutant to tyrosine kinase inhibitors in HER2-positive lung cancer. J Recept Signal Transduct Res. doi:10.3109/10799893.2015.1049361
Vrbic S, Pejcic I, Filipovic S, Kocic B, Vrbic M (2013) Current and future anti-HER2 therapy in breast cancer. J BUON 18:4–16
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng 8:127–134
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Developing and testing of a general amber force field. J Comput Chem 25:1157–1174
Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ (2008) The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 105:2070–2075
Zhou P, Huang J, Tian F (2012) Specific noncovalent interactions at protein-ligand interface: implications for rational drug design. Curr Med Chem 19:226–238
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LY13H160029) and the Key Science and Technology Project of Zhejiang Province (No. 2014C03004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: G. J. Peters.
X. Meng and Y. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Meng, X., Li, Y., Tang, H. et al. Drug response to HER2 gatekeeper T798M mutation in HER2-positive breast cancer. Amino Acids 48, 487–497 (2016). https://doi.org/10.1007/s00726-015-2102-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2102-2