Abstract
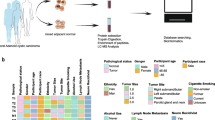
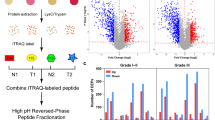
The potential role of protein O-GlcNAcylation in cancer has been studied extensively, and the spread of cancer cells to regional lymph nodes is the first step in the dissemination of breast cancer. However, the correlation between O-GlcNAcylation and lymphatic metastasis in breast cancer remains elusive. In this study, we demonstrated that the overall O-GlcNAcylation as well as O-GlcNAc transferase (OGT) tends to decrease in response to the augmentation of lymph node metastasis (LNM) in invasive ductal breast carcinomas (IDCs). Although accumulating evidence indicates that individual O-GlcNAcylation may be important in the pathogenesis of breast cancer, O-GlcNAcylated proteins in IDCs are still largely unexplored. Herein, O-GlcNAcylated proteins of IDCs were chemo-enzymatically enriched and identified via liquid chromatography combined with tandem mass spectrometry. In total, 155 O-GlcNAcylated proteins were determined, of which 41 were only observed in LNM tissues, while 40 were unique in non-LNM samples. Gene ontology analysis showed that O-GlcNAc is primarily a nucleocytoplasmic post-translational modification, and most enriched functional terms were related to cancer development in both metastatic and non-metastatic IDCs. Moreover, several O-GlcNAcylated proteins involved in glycolysis and its accessory pathway were identified from LNM and non-LNM groups, respectively. These results indicate that the O-GlcNAcylation statuses of individual proteins were independent of the overall O-GlcNAcylation levels of metastatic and non-metastatic IDCs. Aberrant O-GlcNAc modification of these proteins might be associated with LNM progression.






Similar content being viewed by others
Abbreviations
- OGT:
-
O-GlcNAc transferase
- OGA:
-
O-GlcNAcase
- IDC:
-
Invasive ductal breast carcinoma
- LNM:
-
Lymph node metastasis
- LC–MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- O-GlcNAc:
-
O-linked β-D-N-acetylglucosamine
- PPP:
-
Pentose phosphate pathway
- TAMRA:
-
Tetramethylrhodamine
- HSPA6:
-
Heat shock 70 kDa protein 6
- eEF1A:
-
Elongation factor 1-alpha 1
- ENO1:
-
Enolase 1
- FBP1:
-
Fructose-1,6-bisphosphatase 1
- ALDOB:
-
Aldolase B
- TKT:
-
Transketolase
- TALDO1:
-
Transaldolase 1
- TPI1:
-
Triosephosphate isomerase 1
References
Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H (2003) Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci 44(9):3802–3809
Alfaro JF, Gong C-X, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG, Shabanowitz J, Stanley P (2012) Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci 109(19):7280–7285
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30(21):2678–2683
Bombonati A, Sgroi DC (2011) The molecular pathology of breast cancer progression. J Pathol 223(2):308–318
Caldwell S, Jackson S, Shahriari K, Lynch T, Sethi G, Walker S, Vosseller K, Reginato M (2010) Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29(19):2831–2842
Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J (2013) Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics 13(14):2088–2099
Chou C-F, Smith AJ, Omary M (1992) Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem 267(6):3901–3906
Chua B, Ung O, Taylor R, Boyages J (2001) Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg 71(12):723–728
Comer FI, Hart GW (2000) O-Glycosylation of nuclear and cytosolic proteins dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem 275(38):29179–29182
Cunnick G, Jiang W, Gomez K, Mansel R (2002) Lymphangiogenesis and breast cancer metastasis. Histol Histopathol 17(3):863–870
Dash I, Egbeare D, Straiton M, Schwodler K, Taylor G, Goddard D, McIntosh J, Sutton R (2015) The positive sentinel lymph node biopsy-can we predict which patients will benefit from further surgery. J Cancer Res Ther 3:15–19
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane CH, Lempicki RA (2003) DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4(9):1–11
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W (2010) GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 70(15):6344–6351
Hahne H, Sobotzki N, Nyberg T, Helm D, Borodkin VS, van Aalten DM, Agnew B, Kuster B (2013) Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J Proteome Res 12(2):927–936
Harris M, Howell A, Chrissohou M, Swindell R, Hudson M, Sellwood R (1984) A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer 50:23–30
Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446(7139):1017–1022. doi:10.1038/nature05815
Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res 40(D1):D261–D270
Iyer SPN, Hart GW (2003) Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem 278(27):24608–24616
Krześlak A, Forma E, Bernaciak M, Romanowicz H, Bryś M (2012) Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med 12(1):61–65
Langbein S, Frederiks WM, zur Hausen A, Popa J, Lehmann J, Weiss C, Alken P, Coy JF (2008) Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer 122(11):2422–2428
Ma J, Hart GW (2014) O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics 11(1):8
Matsuura A, Ito M, Sakaidani Y, Kondo T, Murakami K, Furukawa K, Nadano D, Matsuda T, Okajima T (2008) O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J Biol Chem 283(51):35486–35495
Omary M, Ku N, Liao J, Price D (1997) Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem 31:105–140
Roquemore EP, Chevrier MR, Cotter RJ, Hart GW (1996) Dynamic O-GlcNAcylation of the small heat shock protein αB-crystallin. Biochemistry 35(11):3578–3586
Sakaidani Y, Nomura T, Matsuura A, Ito M, Suzuki E, Murakami K, Nadano D, Matsuda T, Furukawa K, Okajima T (2011) O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat Commun 2:583
Schultz J, Pils B (2002) Prediction of structure and functional residues for O-GlcNAcase, a divergent homologue of acetyltransferases. FEBS Lett 529(2):179–182
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7(2):192–198
Slawson C, Hart GW (2011) O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 11(9):678–684
Slawson C, Pidala J, Potter R (2001) Increased N-acetyl-β-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetylglucosamine containing proteins. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1537(2):147–157
Slawson C, Copeland R, Hart GW (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35(10):547–555
Srikanth B, Vaidya MM, Kalraiya RD (2010) O-GlcNAcylation determines the solubility, filament organization, and stability of keratins 8 and 18. J Biol Chem 285(44):34062–34071
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Vizcaíno JA, Côté RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res 41(D1):D1063–D1069
Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW (2010) Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal 3(104):ra2
Wang J, Torii M, Liu H, Hart GW, Hu Z-Z (2011) dbOGAP-an integrated bioinformatics resource for protein O-GlcNAcylation. BMC Bioinformatics 12(1):91
Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan Y-T, Jones SP (2010) O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci 107(41):17797–17802
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291(5512):2376–2378
Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, Peters EC, Driggers EM, Hsieh-Wilson LC (2012) Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337(6097):975–980
Zachara NE, Hart GW (2002) The emerging significance of O-GlcNAc in cellular regulation. Chem Rev 102(2):431–438
Zachara NE, Hart GW (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochimica et Biophysica Acta (BBA) Gen Subj 1673(1):13–28
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, Grant No. 2012CB910303, 2012CB910301 and 2011CB910603) and National Natural Science Foundation of China (31470795, 31170768).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: P. R. Jungblut.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, K., Gao, Y., Hou, W. et al. Proteomic analysis of O-GlcNAcylated proteins in invasive ductal breast carcinomas with and without lymph node metastasis. Amino Acids 48, 365–374 (2016). https://doi.org/10.1007/s00726-015-2089-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2089-8