Abstract
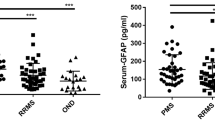
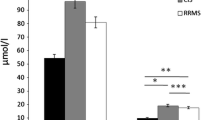
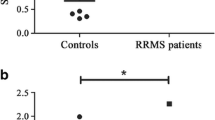
The pathogenic hallmarks of multiple sclerosis (MS) and neuromyelitis optica (NMO) are cellular and humoral inflammatory infiltrates and subsequent demyelination, or astrocytic cell death in NMO, respectively. These processes are accompanied by disruption of the blood–brain barrier as regularly observed by gadolinium enhancement on magnetic resonance imaging. The role of the l-arginine/nitric oxide (NO) pathway in the pathophysiology of neuroinflammatory diseases, such as MS and NMO, remains unclear. In the present study, we measured the concentrations of the nitric oxide (NO) metabolites nitrate and nitrite, the endogenous substrates of NO synthase (NOS) l-arginine (Arg) and l-homoarginine (hArg), and asymmetric dimethylarginine (ADMA), the endogenous inhibitor of NOS activity, in the serum and cerebrospinal fluid (CSF) of patients with MS, NMO or other neurologic diseases (OND). MS (551 ± 23 nM, P = 0.004) and NMO (608 ± 51 nM, P = 0.006) patients have higher ADMA concentrations in serum than healthy controls (HC; 430 ± 24 nM). For MS, this finding was confirmed in CSF (685 ± 100 nM in relapsing–remitting multiple sclerosis, RRMS; 597 ± 51 nM in secondary progressive multiple sclerosis, SPMS) compared with OND (514 ± 37 nM; P = 0.003). Serum concentrations of Arg (61.1 ± 9.7 vs. 63.6 ± 4.9 µM, P = 0.760), hArg (2.62 ± 0.26 vs. 2.52 ± 0.23 µM, P = 0.891), nitrate (38.1 ± 2.2 vs. 38.1 ± 3.0 µM) and nitrite (1.37 ± 0.09 vs. 1.55 ± 0.03 µM) did not differ between MS and OND. Also, CSF concentrations of hArg (0.685 ± 0.100 µM in RRMS, 0.597 ± 0.051 µM in SPMS, 0.514 ± 0.037 µM in OND), nitrate (11.3 ± 0.6 vs. 10.5 ± 0.3 µM) and nitrite (2.84 ± 0.32 vs. 2.41 ± 0.11 µM) did not differ between the groups. In NMO patients, however, serum Arg (117 ± 11 vs. 64 ± 4.9 μM, P = 0.004), nitrate (29 ± 2.1 vs. 38 ± 3 μM, P = 0.03), and nitrite (1.09 ± 0.02 vs. 1.55 ± 0.033 µM, P < 0.0001) were significantly different as compared to OND. Symmetric dimethylarginine (SDMA) concentration did not differ in serum between MS and HC (779 ± 43 vs. 755 ± 58 nM, P = 0.681) or in CSF between MS and OND patients (237 ± 11 vs. 230 ± 17 nM, P = 0.217). Our study suggests a potential role for ADMA and Arg in neuroinflammatory diseases with diverse functions in MS and NMO. Higher ADMA synthesis may explain reduced NO availability in NMO. hArg and SDMA seem not to play an important role in MS and NMO.




Similar content being viewed by others
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine (N G, N G-dimethyl-l-arginine)
- AGAT:
-
Arginine:glycine amidinotransferase
- AQP:
-
Aquaporin
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluids
- GC–MS:
-
Gas chromatography–mass spectrometry
- GC–MS/MS:
-
Gas chromatography–tandem mass spectrometry
- hArg:
-
Homoarginine
- HC:
-
Healthy controls
- MS:
-
Multiple sclerosis
- NMO:
-
Neuromyelitis optica
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- OND:
-
Other neurologic diseases
- RRMS:
-
Relapsing–remitting multiple sclerosis
- SDMA:
-
Symmetric dimethylarginine (N G, N G′-dimethyl-l-arginine)
- SPMS:
-
Secondary progressive multiple sclerosis
References
Atzler D, Rosenberg M, Andersso M, Choe CU, Lutz M, Zugck C et al (2013) Homoarginine—an independent marker of mortality in heart failure. Int J Cardiol 168:4907–4909
Atzler D, Gore MO, Ayers CR, Choe CU, Böger RH, de Lemos JA, McGuire DK, Schwedhelm E (2014) Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler Thromb Vasc Biol 34:2501–2507
Böger RH, Maas R, Schulze F, Schwedhelm E (2009) Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res 60:481–487
Bretscher LE, Li H, Poulos TL, Griffith OW (2003) Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5-(iminoalkenyl)-ornithines. J Biol Chem 278:46789–46797
Choe CU, Atzler D, Wild PS, Carter AM, Böger RH, Ojeda F, Simova O, Stockebrand M, Lackner K, Nabuurs C, Marescau B, Streichert T, Muller C, Luneburg N, De Deyn PP, Benndorf RA, Baldus S, Gerloff C, Blankenberg S, Heerschap A, Grant PJ, Magnus T, Zeller T, Isbrandt D, Schwedhelm E (2013) Homoarginine levels are regulated by l-arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461
Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, März W, Wanner C (2011) Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail 13:852–859
Farias AS, de la Hoz C, Castro FR, Oliveira EC, Ribeiro dos Reis JR, Silva JS, Langone F, Santos LM (2007) Nitric oxide and TNFalpha effects in experimental autoimmune encephalomyelitis demyelination. NeuroImmunoModulation 14:32–38
Haghikia A, Mergia E, Friebe A, Eysel UT, Koesling D, Mittmann T (2007) Long-term potentiation in the visual cortex requires both nitric oxide receptor guanylyl cyclases. J Neurosci 27:818–823
Haghikia A, Hohlfeld R, Gold R, Fugger L (2013) Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol Med 19:309–319
Horowitz JD, Heresztyn T (2007) An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: methodological considerations. J Chromatogr B 851:42–50
Kayacelebi AA, Beckmann B, Gutzki FM, Jordan J, Tsikas D (2014a) GC–MS and GC–MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids 46:2205–2217
Kayacelebi AA, Pham VV, Willers J, Hahn A, Stichtenoth DO, Jordan J, Tsikas D (2014b) Plasma homoarginine (hArg) and asymmetric dimethylarginine (ADMA) in patients with rheumatoid arthritis: is homoarginine a cardiovascular corrective in rheumatoid arthritis, an anti-ADMA? Int J Cardiol 176:1129–1131
Kayacelebi AA, Knöfel AK, Beckmann B, Hanff E, Warnecke G, Tsikas D (2015) Measurement of unlabeled and stable isotope-labeled homoarginine, arginine and their metabolites in biological samples by GC-MS and GC-MS/MS. Amino Acids. doi:10.1007/s00726-015-1984-3
Khalil AA, Tsikas D, Akolekar R, Jordan J, Nicolaides KH (2013) Asymmetric dimethylarginine, arginine and homoarginine at 11–13 weeks’ gestation and preeclampsia: a case-control study. J Hum Hypertens 27:38–43
Kielstein A, Tsikas D, Galloway GP, Mendelson JE (2007) Asymmetric dimethylarginine (ADMA)—a modulator of nociception in opiate tolerance and addiction? Nitric Oxide 17:55–59
Leiper J, Vallance P (1999) Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43:542–548
Leiper JM, Vallance P (2006) The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur J Clin Pharmacol 26:33–38
Ljubisavljevic S, Stevanovic I, Pavlovic R, Sokolovic D, Pavlovic D, Cvetkovic T (2012) J Neurol Sci 318:106–111
Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
May M, Batkai S, Zörner AA, Tsikas D, Jordan J, Engeli S (2014) Clinical evaluation of extracellular ADMA concentrations in human blood and adipose tissue. Int J Mol Sci 15:1189–1200
May M, Kayacelebi AA, Batkai S, Jordan J, Tsikas D, Engeli S (2015) Plasma and tissue homoarginine concentrations in healthy and obese humans. Amino Acids. doi:10.1007/s00726-015-1922-4
Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE (1994) Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience 3:575–585
Moali C, Boucher JL, Sari MA, Stuehr DJ, Mansuy D (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 37:10453–10460
Moali C, Brollo M, Custot J, Sari MA, Boucher JL, Stuehr DJ, Mansuy D (2000) Recognition of alpha-amino acids bearing various C=NOH functions by nitric oxide synthase and arginase involves very different structural determinants. Biochemistry 39:8208–8218
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. New Engl J Med 329:2002–2012
Papadopoulos MC, Bennett JL, Verkman AL (2014) Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 10:493–506
Pilz S, Tomaschitz A, Meinitzer A, Drechsler C, Ritz E, Krane V, Wanner C, Bohm BO, März W (2011) Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 42:1132–1134
Pilz S, Teerlink T, Scheffer PG, Meinitzer A, Rutters F, Tomaschitz A, Drechsler C, Kienreich K, Nijpels G, Stehouwer CD, März W, Dekker JM (2014) Homoarginine and mortality in an older population: the Hoorn study. Eur J Clin Invest 44:200–208
Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A (2015a) Homoarginine in the renal and cardiovascular systems. Amino Acids. doi:10.1007/s00726-015-1993-2
Pilz S, Putz-Bankuti C, Meinitzer A, März W, Kienreich K, Stojakovic T, Pieber TR, Stauber RE (2015b) Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids. doi:10.1007/s00726-015-2000-7
Redjak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RHC, Barkhof F, Polman CH, Uitdehaag BMJ, Giovannoni G (2004) CSF nitrite oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 63:1439–1445
Sandoo A, Dimitroulas T, Hodson J, Smith JP, Douglas KM, Kitas GD (2014) Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: a 6 year follow-up study. Rheumatology (Oxford). doi:10.1093/rheumatology/keu349
Smith KJ, Lassmann H (2002) The role of nitric oxide in multiple sclerosis. Lancet Neurol 1:232–241
Stojanovic I, Vojinovic S, Ljubisavljevic S, Pavlovic R, Basic J, Pavlovic D, Ilic A, Cvetkovic T, Stukalov M (2012) INF-β1b therapy modulates l-arginine and nitric oxide metabolism in patients with relapse remittent multiple sclerosis. J Neurol Sci 323:187–192
Tsikas D (2000) Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 72:4064–4072
Tsikas D (2009) De novo synthesis of trideuteromethyl esters of amino acids for use in GC–MS and GC–tandem MS exemplified for ADMA in human plasma and urine: standardization, validation, comparison and proof of evidence for their aptitude as internal standards. J Chromatogr B 877:2308–2320
Tsikas D (2015) Circulating and excretory nitrite and nitrate: their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide 45C:1–3
Tsikas D, Kayacelebi AA (2014) Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system? Circ J 78:2094–2095
Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC (2000a) Endogenous nitric oxide synthase inhibitors are responsible for the l-arginine paradox. FEBS Lett 478:1–3
Tsikas D, Sandmann J, Savva A, Luessen P, Böger RH, Gutzki FM, Frölich JC (2000b) Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography–mass spectrometry. J Chromatogr B 742:143–153
Tsikas D, Schubert B, Gutzki FM, Sandmann J, Frölich JC (2003) Quantitative determination of circulating and urinary asymmetric dimethylarginine (ADMA) in humans by gas chromatography–tandem mass spectrometry as methyl ester tri(N-pentafluoropropionyl) derivative. J Chromatogr B 798:87–99
Tsikas D, Beckmann B, Gutzki FM, Jordan J (2011) Simultaneous gas chromatography-tandem mass spectrometry quantification of symmetric and asymmetric dimethylarginine in human urine. Anal Biochem 413:60–62
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Conflict of interest
The authors declare that they have no conflict of interest related to the present research.
Ethical standard
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an approval by the Ethics Committee of the Ruhr-University Bochum (Bochum, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haghikia, A., Kayacelebi, A.A., Beckmann, B. et al. Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids 47, 1837–1845 (2015). https://doi.org/10.1007/s00726-015-2015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2015-0